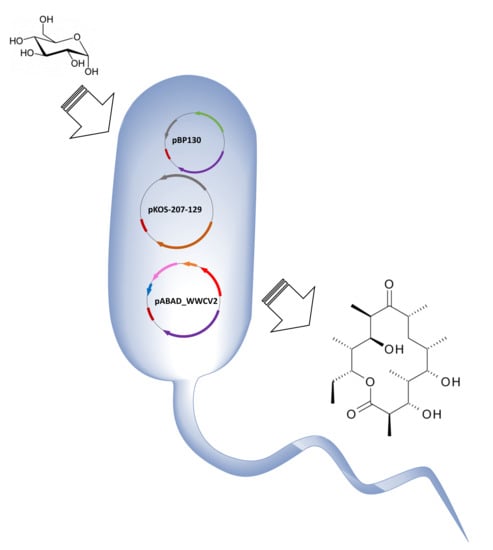
Heterologous Production of 6-Deoxyerythronolide B in Escherichia coli through the Wood Werkman Cycle
Abstract
:1. Introduction
2. Results
2.1. Assembling a Three-Plasmid System for the Production of 6-dEB in E. coli
2.2. Production of 6-dEB in E. coli with Propionate Supplementation
2.3. Production of 6-dEB in E. coli without Propionate Supplementation
3. Discussion
4. Materials and Methods
4.1. Strains and Plasmids
4.2. Media Composition
4.3. Plasmid Construction
4.4. 6-Deoxyerythronolide B Production in Aerobic Conditions
4.5. 6-Deoxyerythronolide B Identification and Quantitation
4.6. 6-dEB Production from Glucose in Anaerobic Conditions
4.7. HPLC Analysis of Organic Acids
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Li, J.; Neubauer, P. Escherichia coli as a cell factory for heterologous production of nonribosomal peptides and polyketides. New Biotechnol. 2014, 31, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Peirú, S.; Menzella, H.G.; Rodríguez, E.; Carney, J.; Gramajo, H. Production of the potent antibacterial polyketide erythromycin C in Escherichia coli. Appl. Environ. Microbiol. 2005, 71, 2539–2547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barajas, J.F.; Blake-Hedges, J.M.; Bailey, C.B.; Curran, S.; Keasling, J.D. Engineered polyketides: Synergy between protein and host level engineering. Synth. Syst. Biotechnol. 2017, 2, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Boghigian, B.A.; Armando, J.; Pfeifer, B.A. Methods and options for the heterologous production of complex natural products. Nat. Prod. Rep. 2011, 28, 125–151. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Pfeifer, B.A. Bacterial hosts for natural product production. Mol. Pharm. 2008, 5, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Fujii, I. Heterologous expression systems for polyketide synthases. Nat. Prod. Rep. 2009, 26, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Y.; Tang, Y. Engineered biosynthesis of bacterial aromatic polyketides in Escherichia coli. PNAS 2008, 105, 20683–20688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeifer, B.A.; Admiraal, S.J.; Gramajo, H.; Cane, D.E.; Khosla, C. Biosynthesis of complex polyketides in a metabolically engineered strain of E. coli. Science 2001, 291, 1790–1792. [Google Scholar] [CrossRef] [PubMed]
- Robbins, T.; Liu, Y.C.; Cane, D.E.; Khosla, C. Structure and mechanism of assembly line polyketide synthases. Curr. Opin. Struct. Biol. 2016, 41, 10–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeifer, B.; Hu, Z.; Licari, P.; Khosla, C. Process and metabolic strategies for improved production of Escherichia coli-derived 6-deoxyerythronolide B. Appl. Environ. Microbiol. 2002, 68, 3287–3292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murli, S.; Kennedy, J.; Dayem, L.C.; Carney, J.R.; Kealey, J.T. Metabolic engineering of Escherichia coli for improved 6-deoxyerythronolide B production. J. Ind. Microbiol. Biotechnol. 2003, 30, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.; Tran, C.; Licari, P.; Galazzo, J. Development of a high cell-density fed-batch bioprocess for the heterologous production of 6-deoxyerythronolide B in Escherichia coli. J. Biotechnol. 2004, 110, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Vandova, G.A.; O’Brien, R.V.; Lowry, B.; Robbins, T.F.; Fischer, C.R.; Davis, R.W.; Khosla, C.; Harvey, C.J.; Hillenmeyer, M.E. Heterologous expression of diverse propionyl-CoA carboxylases affects polyketide production in Escherichia coli. J. Antibiot. 2017, 70, 859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, M.; Pfeifer, B.A. Metabolic and pathway engineering to influence native and altered erythromycin production through E. coli. Met. Eng. 2013, 19, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Garcia, R.A.; McCubbin, T.; Turner, M.S.; Nielsen, L.K.; Marcellin, E. Engineering Escherichia coli for propionic acid production through the Wood–Werkman cycle. Biotechnol. Bioeng. 2019, 1–17. [Google Scholar] [CrossRef]
- González-Lergier, J.; Broadbelt, L.J.; Hatzimanikatis, V. Analysis of the maximum theoretical yield for the synthesis of erythromycin precursors in Escherichia coli. Biotechnol. Bioeng. 2006, 95, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Kumpfmüller, J.; Methling, K.; Fang, L.; Pfeifer, B.A.; Lalk, M.; Schweder, T. Production of the polyketide 6-deoxyerythronolide B in the heterologous host Bacillus subtilis. Appl. Microbiol. Biotechnol. 2016, 100, 1209–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Conditions | Relevant Genotype | Glucose 1,2,* (mM) | Propionate 3 (mM) | 6-dEB 4,* (mg/L) | 6-dEB Molar Yield 5 |
|---|---|---|---|---|---|
| SM, aerobic | E. coli 207-3/pBR322/pKOS207-129e | 26.9 ± 3.6 | 10 | ND | - |
| CDM, aerobic | E. coli 207-3/pBR322/pKOS207-129e | 105.9 ± 6.6 | 10 | ND | - |
| CDM, anaerobic | E. coli 207-3/pBR322/pKOS207-129e | 56.6 ± 2.89 | - | ND | - |
| CDM, anaerobic | E. coli 207-3/pBR322/pKOS207-129e/pACYC133 | 53.9 ± 3.8 | - | ND | - |
| SM, aerobic | E. coli 207-3/pBP130/pKOS207-129 | 19 ± 3.6 | 10 | 6.24 ± 1.74 | 1.71 × 10−3 |
| CDM, aerobic | E. coli 207-3/pBP130/pKOS207-129 | 110 ± 9.6 | 10 | 2.68 ± 0.16 | 7.33 × 10−4 |
| CDM, anaerobic | E. coli 207-3/pBP130/pKOS207-129 | 57.3 ± 3.7 | - | ND | - |
| CDM, anaerobic | E. coli 207-3/pBP130/pKOS207-129/pABAD_WWCV2 | 55.7 ± 5.45 | - | 0.81 ± 0.08 | 3.98 × 10−5 |
| Strain/Plasmid | Description | Reference |
|---|---|---|
| Strains | ||
| E. coli DH5α | Cloning host | Bioline (Aust) Pty Ltd., Alexandria, NSW, Australia |
| E. coli 207-3 | K173-145 ygfG::T7prom-accA1-T7prom-pccB-T7term | [13] |
| Plasmids | ||
| pBP130 | bla, T7prom-DEBS 2-ribosome binding site-DEBS3-T7term, ColE1 ori | [10] |
| pKOS-207_129 | stp, T7prom-DEBS1-T7term, RSF1010 ori | [13] |
| pBAD_WWCV2 | pBR322 derived plasmid. bla, araC-PBAD - mutA-mutB-mce- mtcA-mtcB-mtcC-mtcD-pst-tT7, ColE1 ori | [17] |
| pACYC133 | cat, tet, p15A-ori | New England Biolabs, Ipswich, Massachusetts, MA, USA |
| pABAD_WWCV2 | cat, tet, p15A-ori, araC-PBAD - mutA-mutB-mce- mtcA-mtcB-mtcC-mtcD-pst-tT7, p15A-ori | This work |
| pBR322 | bla, ColE1 ori | New England Biolabs, Ipswich, Massachusetts, MA, USA |
| pKOS-207_129e | stp, RSF1010 ori | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Garcia, R.A.; Nielsen, L.K.; Marcellin, E. Heterologous Production of 6-Deoxyerythronolide B in Escherichia coli through the Wood Werkman Cycle. Metabolites 2020, 10, 228. https://doi.org/10.3390/metabo10060228
Gonzalez-Garcia RA, Nielsen LK, Marcellin E. Heterologous Production of 6-Deoxyerythronolide B in Escherichia coli through the Wood Werkman Cycle. Metabolites. 2020; 10(6):228. https://doi.org/10.3390/metabo10060228
Chicago/Turabian StyleGonzalez-Garcia, R. Axayacatl, Lars K. Nielsen, and Esteban Marcellin. 2020. "Heterologous Production of 6-Deoxyerythronolide B in Escherichia coli through the Wood Werkman Cycle" Metabolites 10, no. 6: 228. https://doi.org/10.3390/metabo10060228
APA StyleGonzalez-Garcia, R. A., Nielsen, L. K., & Marcellin, E. (2020). Heterologous Production of 6-Deoxyerythronolide B in Escherichia coli through the Wood Werkman Cycle. Metabolites, 10(6), 228. https://doi.org/10.3390/metabo10060228