Urine Metabolome during Parturition
Abstract
:1. Introduction
2. Results
2.1. Urine Metabolomic Study of Labor
2.2. Analysis of Metabolic Pattern
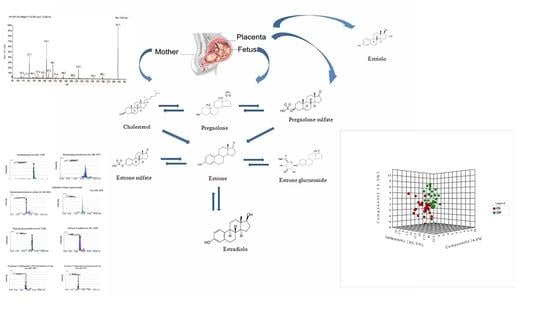
2.3. Steroid Hormone
2.4. Amino Acid
3. Discussion
4. Materials and Methods
4.1. Subjects and Urine Collection
4.2. Metabolite Extraction aHILIC-UHPLC Separation
4.3. Mass Spectrometry
4.4. Data Elaboration and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buckley, S.J. Executive Summary of Hormonal Physiology of Childbearing: Evidence and Implications for Women, Babies, and Maternity Care. J. Périnat. Educ. 2015, 24, 145–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Care in Normal Birth: A Practical Guide. Geneva: Maternal and Newborn Health/Safe Moderhood Unit, Family and Reproductive Health. Published 1996. Available online: http://apps.who.int/iris/bitstream/10665/63167/1/WHO_FRH_MSM_96.24.pdf (accessed on 16 May 2020).
- Australian Institute of Health and Welfare. Aust. Coll. Midwives Inc. J. 1995, 8, 18. [CrossRef]
- Ministero della salute. Rapporto Annuale Sull’attività di Ricovero Ospedaliero. 2007. Available online: http://www.salute.gov.it/imgs/c_17_pubblicazioni_1152_allegato.pdf (accessed on 16 May 2020).
- Heng, Y.J.; Pennell, C.E.; Chua, H.N.; Perkins, J.E.; Lye, S.J. Whole Blood Gene Expression Profile Associated with Spontaneous Preterm Birth in Women with Threatened Preterm Labor. PLoS ONE 2014, 9, e96901. [Google Scholar] [CrossRef] [PubMed]
- Veras, T.C.D.S.; Mathias, T.A.D.F. Hospitalizations leading causes for maternal disorders. Rev. Esc. Enferm. USP 2014, 48, 401–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezold, K.Y.; Karjalainen, M.K.; Hallman, M.; Teramo, K.; Muglia, L.J. The genomics of preterm birth: From animal models to human studies. Genome Med. 2013, 5, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanley, G.E.; Munro, S.; Greyson, D.; Gross, M.M.; Hundley, V.; Spiby, H.; Janssen, P. Diagnosing onset of labor: A systematic review of definitions in the research literature. BMC Pregnancy Childbirth 2016, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollier, L.P.; A Keelan, J.; Hickey, M.; Maybery, M.; Whitehouse, A.J.O. Measurement of Androgen and Estrogen Concentrations in Cord Blood: Accuracy, Biological Interpretation, and Applications to Understanding Human Behavioral Development. Front. Endocrinol. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, B.F.; Taggart, M. Are animal models relevant to key aspects of human parturition? Am. J. Physiol. Integr. Comp. Physiol. 2009, 297, R525–R545. [Google Scholar] [CrossRef] [Green Version]
- Graça, G.; Duarte, I.F.; Barros, A.; Goodfellow, B.; Diaz, S.; Carreira, I.M.; Couceiro, A.B.; Galhano, E.; Gil, A.M. 1H NMR Based Metabonomics of Human Amniotic Fluid for the Metabolic Characterization of Fetus Malformations. J. Proteome Res. 2009, 8, 4144–4150. [Google Scholar] [CrossRef]
- Caboni, P.; Meloni, A.; Lussu, M.; Carta, E.; Barberini, L.; Noto, A.; Deiana, S.F.; Mereu, R.; Ragusa, A.; Paoletti, A.M.; et al. Urinary metabolomics of pregnant women at term: A combined GC/MS and NMR approach. J. Matern. Neonatal Med. 2014, 27, 4–12. [Google Scholar] [CrossRef]
- Meloni, A.; Palmas, F.; Barberini, L.; Mereu, R.; Deiana, S.F.; Fais, M.F.; Noto, A.; Fattuoni, C.; Mussap, M.; Ragusa, A.; et al. PROM and Labour Effects on Urinary Metabolome: A Pilot Study. Dis. Markers 2018, 2018, 1042479. [Google Scholar] [CrossRef] [PubMed]
- Hemström, P.; Irgum, K. Hydrophilic interaction chromatography. J. Sep. Sci. 2006, 29, 1784–1821. [Google Scholar] [CrossRef] [PubMed]
- Alpert, A.J. Electrostatic Repulsion Hydrophilic Interaction Chromatography for Isocratic Separation of Charged Solutes and Selective Isolation of Phosphopeptides. Anal. Chem. 2008, 80, 62–76. [Google Scholar] [CrossRef] [Green Version]
- Clasquin, M.F.; Melamud, E.; Rabinowitz, J.D. LC-MS Data Processing with MAVEN: A Metabolomic Analysis and Visualization Engine. Curr. Protoc. Bioinform. 2012, 37. [Google Scholar] [CrossRef] [Green Version]
- Glass, I.A.; Lam, R.C.; Chang, T.; Roitman, E.; Shapiro, L.J.; Shackleton, C.H. Steroid sulphatase deficiency is the major cause of extremely low oestriol production at mid-pregnancy: A urinary steroid assay for the discrimination of steroid sulphatase deficiency from other causes. Prenat. Diagn. 1998, 18, 789–800. [Google Scholar] [CrossRef]
- Peltoketo, H.; Luu-The, V.; Simard, J.; Adamski, J. 17beta-hydroxysteroid dehydrogenase (HSD)/17-ketosteroid reductase (KSR) family; nomenclature and main characteristics of the 17HSD/KSR enzymes. J. Mol. Endocrinol. 1999, 23, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Fabregat, A.; Marcos, J.; Garrostas, L.; Segura, J.; Pozo, O.; Ventura, R. Evaluation of urinary excretion of androgens conjugated to cysteine in human pregnancy by mass spectrometry. J. Steroid Biochem. Mol. Boil. 2014, 139, 192–200. [Google Scholar] [CrossRef]
- Coughtrie, M.W.H.; Sharp, S.; Maxwell, K.; Innes, N.P. Biology and function of the reversible sulfation pathway catalysed by human sulfotransferases and sulfatases. Chem. Interact. 1998, 109, 3–27. [Google Scholar] [CrossRef]
- Zhu, B.T. Functional role of estrogen metabolism in target cells: Review and perspectives. Carcinogenesis 1998, 19, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Marcos, J.; Craig, W.Y.; Palomaki, G.E.; Kloza, E.M.; Haddow, J.E.; Roberson, M.; Bradley, L.A.; Shackleton, C. Maternal urine and serum steroid measurements to identify steroid sulfatase deficiency (STSD) in second trimester pregnancies. Prenat. Diagn. 2009, 29, 771–780. [Google Scholar] [CrossRef]
- Christensen, P.J.; Date, J.W.; Schønheyder, F.; Volqvartz, K. Amino Acids in Blood Plasma and Urine During Pregnancy. Scand. J. Clin. Lab. Investig. 1957, 9, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.A. Lipid Metabolism in Pregnancy and its Consequences in the Fetus and Newborn. Endocrine 2002, 19, 43–56. [Google Scholar] [CrossRef]
- Kalhan, S.C.; Rossi, K.Q.; Gruca, L.L.; Super, D.M.; Savin, S.M. Relation between transamination of branched-chain amino acids and urea synthesis: Evidence from human pregnancy. Am. J. Physiol. Content 1998, 275, E423–E431. [Google Scholar] [CrossRef]
- Cetin, I.; Fennessey, P.V.; Quick, A.N.; Marconi, A.M.; Meschia, G.; Battaglia, F.C.; Sparks, J.W. Glycine turnover and oxidation and hepatic serine synthesis from glycine in fetal lambs. Am. J. Physiol. Metab. 1991, 260, E371–E378. [Google Scholar] [CrossRef] [PubMed]
- Cetin, I.; Marconi, A.M.; Bozzetti, P.; Sereni, L.P.; Corbetta, C.; Pardi, G.; Battaglia, F.C. Umbilical amino acid concentrations in appropriate and small for gestational age infants: A biochemical difference present in utero. Am. J. Obstet. Gynecol. 1988, 158, 120–126. [Google Scholar] [CrossRef]
- Hayashi, S.; Sanada, K.; Sagawa, N.; Yamada, N.; Kido, K. Umbilical Vein-Artery Differences of Plasma Amino Acids in the Last Trimester of Human Pregnancy. Neonatology 1978, 34, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Ronzoni, S.; Marconi, A.M.; Cetin, I.; Paolini, C.L.; Teng, C.; Pardi, G.; Battaglia, F.C. Umbilical amino acid uptake at increasing maternal amino acid concentrations: Effect of a maternal amino acid infusate. Am. J. Obstet. Gynecol. 1999, 181, 477–483. [Google Scholar] [CrossRef]
- Cetin, I.; Fennessey, P.V.; Sparks, J.W.; Meschia, G.; Battaglia, F.C. Fetal serine fluxes across fetal liver, hindlimb, and placenta in late gestation. Am. J. Physiol. Metab. 1992, 263, E786–E793. [Google Scholar] [CrossRef]
- Snell, K. Liver enzymes of serine metabolism during neonatal development of the rat. Biochem. J. 1980, 190, 451–455. [Google Scholar] [CrossRef] [Green Version]
- Snell, K. Enzymes of serine metabolism in normal, developing and neoplastic rat tissues. Adv. Enzym. Regul. 1984, 22, 325–400. [Google Scholar] [CrossRef]
- Snell, K.; Natsumeda, Y.; Wéber, G. The modulation of serine metabolism in hepatoma 3924A during different phases of cellular proliferation in culture. Biochem. J. 1987, 245, 609–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battaglia, F.C. Glutamine and glutamate exchange between the fetal liver and the placenta. J. Nutr. 2000, 130, 974S–977S. [Google Scholar] [CrossRef] [PubMed]
- Holzman, I.R.; A Lemons, J.; Meschia, G.; Battaglia, F.C. Uterine uptake of amino acids and placental glutamine--glutamate balance in the pregnant ewe. J. Dev. Physiol. 1979, 1, 137–149. [Google Scholar] [PubMed]
- Liechty, E.A.; Kelley, J.; Lemons, J.A. Effect of Fasting on Uteroplacental Amino Acid Metabolism in the Pregnant Sheep. Neonatology 1991, 60, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Marconi, A.M.; Battaglia, F.C.; Meschia, G.; Sparks, J.W. A comparison of amino acid arteriovenous differences across the liver and placenta of the fetal lamb. Am. J. Physiol. Metab. 1989, 257, E909–E915. [Google Scholar] [CrossRef]
- Moores, R.R.; Vaughn, P.R.; Battaglia, F.C.; Fennessey, P.V.; Wilkening, R.B.; Meschia, G. Glutamate metabolism in fetus and placenta of late-gestation sheep. Am. J. Physiol. Integr. Comp. Physiol. 1994, 267, R89–R96. [Google Scholar] [CrossRef]
- Loy, G.L.; Quick, A.N.; Hay, W.W.; Meschia, G.; Battaglia, F.C.; Fennessey, P.V. Fetoplacental deamination and decarboxylation of leucine. Am. J. Physiol. Metab. 1990, 259, E492–E497. [Google Scholar] [CrossRef]
- Vaughn, P.R.; Lobo, C.; Battaglia, F.C.; Fennessey, P.V.; Wilkening, R.B.; Meschia, G. Glutamine-glutamate exchange between placenta and fetal liver. Am. J. Physiol. Metab. 1995, 268, E705–E711. [Google Scholar] [CrossRef]
- Hay, W.W.; Molina, R.A.; Digiacomo, J.E.; Meschia, G. Model of placental glucose consumption and glucose transfer. Am. J. Physiol. Integr. Comp. Physiol. 1990, 258, R569–R577. [Google Scholar] [CrossRef]
- Apatu, R.S.; Barnes, R.J. Blood flow to and the metabolism of glucose and lactate by the liver in vivo in fetal, newborn and adult sheep. J. Physiol. 1991, 436, 431–447. [Google Scholar] [CrossRef]
- Gevi, F.; Zolla, L.; Gabriele, S.; Persico, A. Urinary metabolomics of young Italian autistic children supports abnormal Tryptophan and purine metabolism. Mol. Autism 2016, 7, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timperio, A.M.; D’Amici, G.M.; Zolla, L. Analysis of the Cattle Liver Proteome by Highensitive Liquid Chromatography Coupled with Mass Spectrometry Method. Liver Proteomics 2012, 4, 3–62. [Google Scholar] [CrossRef]
- Melamud, E.; Vastag, L.; Rabinowitz, J.D. Metabolomic Analysis and Visualization Engine for LC−MS Data. Anal. Chem. 2010, 82, 9818–9826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, J.; Mandal, R.; Sinelnikov, I.V.; Broadhurst, D.; Wishart, D.S. MetaboAnalyst 2.0—A comprehensive server for metabolomic data analysis. Nucleic Acids Res. 2012, 40, W127–W133. [Google Scholar] [CrossRef] [Green Version]
- Dettmer, K.; Aronov, P.A.; Hammock, B.D. Mass spectrometry-based metabolomics. Mass Spectrom. Rev. 2006, 26, 51–78. [Google Scholar] [CrossRef]
- Ellis, D.I.; Goodacre, R. Metabolic fingerprinting in disease diagnosis: Biomedical applications of infrared and Raman spectroscopy. Analyst 2006, 131, 875. [Google Scholar] [CrossRef]


| Estrogens | Molecular Weight | Detection Mode | Out of Labor | Active Phase | Retention Time | % |
|---|---|---|---|---|---|---|
| 3 hydroxy2-methyl-1H-quinolin-one | 175.05 | Positive | 9300 | 95,000 | 7:00 | 3% ↑ |
| 19 chloro19-Chloro-3beta-hydroxyandrost-5-en-17-one = dehydroepiandrosterone | 365.1695 | Positive | 20000 | 39,000 | 5:0035 | 95% ↑ |
| Androst-5-ene-3beta,17beta-diol = androsterone | 290.1736 | Positive | 28,000 | 22,000 | 7:50 | 21% ↓ |
| Androsterone | 290.1736 | Positive | 28,000 | 22,000 | 7:50 | 21% ↓ |
| Dehydroepiandrosterone Sulfate | 369.0992 | Positive | 39,000 | 31,00 | 5:05 | 92% ↓ |
| Dehydroepiandrosterone | 288.1873 | Positive | 27,000 | 26,000 | 7:30 | 3% ↓ |
| Pregnanediol | 321.2145 | Positive | 8000 | 6000 | 25% ↓ | |
| 3-Hydroxy-1-methylestra-1,3,5(10),6-tetraen-17-one | 283.1551 | Negative | 30,000 | 11,000 | 5:50 | 63% ↑ |
| Tetrahydrocortisone | 365.1592 | Positive | 140,000 | 16,000 | 6:30 | 88% ↓ |
| Estrone 3 sulfate | 351.1085 | Positive | 13,800 | 13,000 | 11:00 | >100% ↑ |
| Estrone gluconoride | 447.1790 | Positive | 79,000 | 10,000 | 12:30 | 87% ↓ |
| Estradiol 17 beta 3 gluconoride | 449.1945 | Positive | 14,000 | 20,000 | 12:00 | 42% ↑ |
| Glucogenic Amino Acid | Molecular Weight | Detection Mode | Out of Labor | Active Phase | Retention Time | % |
|---|---|---|---|---|---|---|
| Ser | 105.09 | Negative | 3400 | 1400 | 12:02 | 59% ↓ |
| Val | 117.15 | Positive | 43,000 | 66,000 | 6:00 | 35% ↑ |
| His | 155.15 | Negative | 72,000 | 94,000 | 13:04 | 23% ↑ |
| Arg | 174.20 | Negative | 1542 | 1742 | 14:00 | 11% ↑ |
| Cys | 121.16 | Negative | 1249 | 2383 | 15:50 | 47% ↑ |
| Ala | 89.09 | Positive | 3400 | 1600 | 20:02 | 47% ↓ |
| Glu | 147.13 | Negative | 1800 | 12,000 | 4:02 | 85% ↑ |
| Gln | 146.14 | Positive | 61,000 | 82,000 | 21:00 | 25% ↑ |
| Ketogenic Amino Acid | Molecular Weight | Detection Mode | Out of Labor | Active Phase | Retention Time | % |
| Leu | 131.17 | Positive | 16,000 | 26,000 | 3:50 | 38% ↑ |
| Lys | 146.19 | Negative | 3900 | 9700 | 19:00 | 60% ↑ |
| Glucogenic and Ketonic | Molecular Weight | Detection Mode | Out of Labor | Active Phase | Retention Time | % |
| Ile | 131.17 | Positive | 16,000 | 26,000 | 12:00 | 38% ↑ |
| Thr | 119.12 | Positive | 5600 | 6800 | 18:00 | 18% ↑ |
| Phe | 165.19 | Positive | 180,000 | 160,000 | 17:00 | 11% ↓ |
| Tyr | 181.19 | Positive | 170,000 | 150,000 | 5:00 | 12% ↓ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gevi, F.; Meloni, A.; Mereu, R.; Lelli, V.; Chiodo, A.; Ragusa, A.; Timperio, A.M. Urine Metabolome during Parturition. Metabolites 2020, 10, 290. https://doi.org/10.3390/metabo10070290
Gevi F, Meloni A, Mereu R, Lelli V, Chiodo A, Ragusa A, Timperio AM. Urine Metabolome during Parturition. Metabolites. 2020; 10(7):290. https://doi.org/10.3390/metabo10070290
Chicago/Turabian StyleGevi, Federica, Alessandra Meloni, Rossella Mereu, Veronica Lelli, Antonella Chiodo, Antonio Ragusa, and Anna Maria Timperio. 2020. "Urine Metabolome during Parturition" Metabolites 10, no. 7: 290. https://doi.org/10.3390/metabo10070290
APA StyleGevi, F., Meloni, A., Mereu, R., Lelli, V., Chiodo, A., Ragusa, A., & Timperio, A. M. (2020). Urine Metabolome during Parturition. Metabolites, 10(7), 290. https://doi.org/10.3390/metabo10070290