Total vs. Bioavailable: Determining a Better 25(OH)D Index in Association with Bone Density and Muscle Mass in Postmenopausal Women
Abstract
:1. Introduction
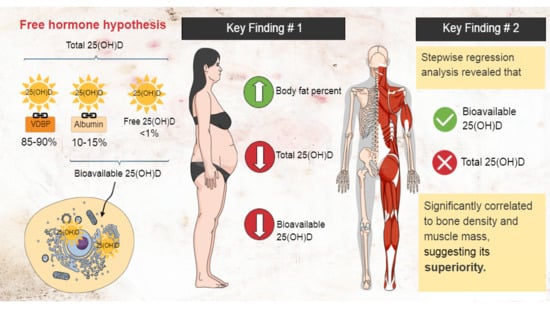
Free Hormone Hypothesis: Total vs. Bioavailable 25(OH)D
2. Results
3. Discussion
3.1. Vitamin D and Bone Density
3.2. Vitamin D and Muscle Mass
4. Materials and Methods
4.1. Selection and Recruitment of Participants
4.2. Demographic Status
4.3. Anthropometric and Obesity Indices Measurements
4.3.1. Height
4.3.2. Body Fat Percentage and Body Mass Index
4.3.3. Waist Circumference
4.4. Bone Density Index Measurement
Quantitative Ultrasound (QUS) Bone Assessments
4.5. Muscle Mass Indices Measurements
Appendicular Skeletal Muscle Mass Index (appSMMI)
4.6. Muscle Strength
4.7. Functional Performance
4.7.1. Short Physical Performance Battery (SPPB) Test
4.7.1.1. One-Leg Stance
4.7.1.2. Gait Speed
4.7.1.3. Sit-to-Stand Chair Test
4.8. Laboratory Measurements
4.9. Statistical Analysis
5. Conclusions
5.1. Limitations
5.2. Strengths
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laurent, M.R.; Dubois, V.; Claessens, F.; Verschueren, S.M.; Vanderschueren, D.; Gielen, E.; Jardí, F. Muscle-bone interactions: From experimental models to the clinic? A critical update. Mol. Cell Endocrinol. 2016, 432, 14–36. [Google Scholar] [CrossRef]
- Bruyère, O.; Cavalier, E.; Reginster, J.Y. Vitamin D and osteosarcopenia: An update from epidemiological studies. Curr. Opin. Clin. Nutr. Metab. Care. 2017, 20, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Sadat-Ali, M.; Al Elq, A.H.; Al-Turki, H.A.; Al-Mulhim, F.A.; Al-Ali, A.K. Influence of vitamin D levels on bone mineral density and osteoporosis. Ann. Saudi Med. 2011, 31, 602–608. [Google Scholar] [CrossRef]
- Cheng, S.; Massaro, J.M.; Fox, C.S.; Larson, M.G.; Keyes, M.J.; McCabe, E.L.; Robins, S.J.; O’Donnell, C.J.; Hoffmann, U.; Jacques, P.F.; et al. Adiposity, cardiometabolic risk, and vitamin D status: The Framingham Heart Study. Diabetes 2010, 59, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Earthman, C.P.; Beckman, L.M.; Masodkar, K.; Sibley, S.D. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: Considerations and implications. Int. J. Obes. 2012, 36, 387–396. [Google Scholar] [CrossRef] [Green Version]
- Grethen, E.; McClintock, R.; Gupta, C.E.; Jones, R.; Cacucci, B.M.; Diaz, D.; Fulford, A.D.; Perkins, S.M.; Considine, R.V.; Peacock, M. Vitamin D and hyperparathyroidism in obesity. J. Clin. Endocrinol. Metab. 2011, 96, 1320–1326. [Google Scholar] [CrossRef]
- Roth, S.M.; Zmuda, J.M.; Cauley, J.A.; Shea, P.R.; Ferrell, R.E. Vitamin D receptor genotype is associated with fat-free mass and sarcopenia in elderly men. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Scott, D.; Blizzard, L.; Fell, J.; Ding, C.; Winzenberg, T.; Jones, G. A prospective study of the associations between 25-hydroxy-vitamin D, sarcopenia progression and physical activity in older adults. Clin. Endocrinol. 2010, 73, 581–587. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.; Kye, S.; Chung, Y.S.; Lee, O. Association of serum vitamin D with osteosarcopenic obesity: Korea National Health and Nutrition Examination Survey 2008–2010. J. Cachexia Sarcopenia Muscle. 2017, 8, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Rosendahl-Riise, H.; Spielau, U.; Ranhoff, A.H.; Gudbrandsen, O.A.; Dierkes, J. Vitamin D supplementation and its influence on muscle strength and mobility in community-dwelling older persons: A systematic review and meta-analysis. J. Hum. Nutr. Diet. 2017, 30, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Kotlarczyk, M.P.; Perera, S.; Ferchak, M.A.; Nace, D.A.; Resnick, N.M.; Greenspan, S.L. Vitamin D deficiency is associated with functional decline and falls in frail elderly women despite supplementation. Osteoporos. Int. 2017, 28, 1347–1353. [Google Scholar] [CrossRef]
- Al-Eisa, E.S.; Alghadir, A.H.; Gabr, S.A. Correlation between vitamin D levels and muscle fatigue risk factors based on physical activity in healthy older adults. Clin. Interv. Aging 2016, 11, 513–522. [Google Scholar]
- Hirani, V.; Cumming, R.G.; Naganathan, V.; Blyth, F.; Le Couteur, D.G.; Hsu, B.; Handelsman, D.J.; Waite, L.M.; Seibel, M.J. Longitudinal Associations Between Vitamin D Metabolites and Sarcopenia in Older Australian men: The Concord Health and Aging in Men Project. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 73, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Dusso, A.S.; Brown, A.J.; Slatopolsky, E. Vitamin D. Am. J. Physiol. Renal Physiol. 2005, 289, F8–F28. [Google Scholar] [CrossRef]
- Bikle, D.D.; Gee, E.; Halloran, B.; Kowalski, M.A.; Ryzen, E.; Haddad, J.G. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J. Clin. Endocrinol. Metab. 1986, 63, 954–959. [Google Scholar] [CrossRef]
- Brown, A.J.; Coyne, D.W. Bioavailable vitamin D in chronic kidney disease. Kidney Int. 2012, 82, 5–7. [Google Scholar] [CrossRef] [Green Version]
- Yousefzadeh, P.; Shapses, S.A.; Wang, X. Vitamin D Binding Protein Impact on 25-Hydroxyvitamin D Levels under Different Physiologic and Pathologic Conditions. Int. J. Endocrinol. 2014, 2014, 98158. [Google Scholar] [CrossRef]
- Bhan, I.; Powe, C.E.; Berg, A.H.; Ankers, E.; Wenger, J.B.; Karumanchi, S.A.; Thadhani, R.I. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney Int. 2012, 82, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Lowe, N.M.; Mitra, S.R.; Foster, P.C.; Bhojani, I.; McCann, J.F. Vitamin D status and markers of bone turnover in Caucasian and South Asian postmenopausal women living in the UK. Br. J. Nutr. 2010, 103, 1706–1710. [Google Scholar] [CrossRef]
- Powe, C.E.; Ricciardi, C.; Berg, A.H.; Erdenesanaa, D.; Collerone, G.; Ankers, E.; Wenger, J.; Karumanchi, S.A.; Thadhani, R.; Bhan, I. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J. Bone Miner. Res. 2011, 26, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. The Lancet 2004, 363, 157–163. [Google Scholar]
- World Health Organization (WHO). Waist Circumference and Waist-Hip Ratio. In Report of WHO Expert Consultation; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Ilich, J.Z.; Kelly, O.J.; Inglis, J.E. Osteosarcopenic Obesity Syndrome: What Is It and How Can It Be Identified and Diagnosed? Curr. Gerontol. Geriatr. Res. 2016, 2016, 7325973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.; Chen, L.; Hsu, P.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Johansen, A.; Evans, W.; Stone, M. Bone assessment in elderly women: What does a low bone ultrasound result tell us about bone mineral density? Arch. Gerontol. Geriatr. 1999, 28, 239–246. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin, D; The National Academic Press USA of Medicine: Washington, DC, USA, 2011. [Google Scholar]
- Powe, C.E.; Seely, E.W.; Rana, S.; Bhan, I.; Ecker, J.; Karumanchi, S.A.; Thadhani, R. First trimester vitamin D, vitamin D binding protein, and subsequent preeclampsia. Hypertension 2010, 56, 758–763. [Google Scholar] [CrossRef] [Green Version]
- Sai, A.J.; Walters, R.W.; Fang, X.; Gallagher, J.C. Relationship between vitamin D, parathyroid hormone, and bone health. J. Clin. Endocrinol. Metab. 2011, 96, E436–E446. [Google Scholar] [CrossRef] [Green Version]
- Rosen, C.J.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; Kovacs, C.S.; et al. IOM committee members respond to Endocrine Society vitamin D guideline. J. Clin. Endocrinol. Metab. 2012, 97, 1146–1152. [Google Scholar] [CrossRef]
- Matchar, D.B.; Chei, C.L.; Yin, Z.X.; Koh, V.; Chakraborty, B.; Shi, X.M.; Zeng, Y. Vitamin D Levels and the Risk of Cognitive Decline in Chinese Elderly People: The Chinese Longitudinal Healthy Longevity Survey. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1363–1368. [Google Scholar] [CrossRef] [Green Version]
- Bischoff-Ferrari, H.A.; Can, U.; Staehelin, H.B.; Platz, A.; Henschkowski, J.; Michel, B.A.; Dawson-Hughes, B.; Theiler, R. Severe vitamin D deficiency in Swiss hip fracture patients. Bone 2008, 42, 597–602. [Google Scholar] [CrossRef]
- Thambiah, S.C.; Wong, T.H.; Gupta, E.D.; Radhakrishnan, A.K.; Gun, S.C.; Chembalingam, G.; Yeap, S.S. Calculation of free and bioavailable vitamin D and its association with bone mineral density in Malaysian women. Malays. J. Pathol. 2018, 40, 287–294. [Google Scholar] [PubMed]
- Steingrimsdottir, L.; Gunnarsson, O.; Indridason, O.S.; Franzson, L.; Sigurdsson, G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA 2005, 294, 2336–2341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souberbielle, J.C.; Cormier, C.; Kindermans, C.; Gao, P.; Cantor, T.; Forette, F.; Baulieu, E.E. Vitamin D status and redefining serum parathyroid hormone reference range in the elderly. J. Clin. Endocrinol. Metab. 2001, 86, 3086–3090. [Google Scholar] [CrossRef]
- Shafinaz, I.S.; Moy, F.M. Vitamin D level and its association with adiposity among multi-ethnic adults in Kuala Lumpur, Malaysia: A cross sectional study. BMC Public Health. 2016, 16, 232. [Google Scholar] [CrossRef] [Green Version]
- Lavie, C.J.; DiNicolantonio, J.J.; Milani, R.V.; O’Keefe, J.H. Vitamin D and Cardiovascular Health. Circulation 2013, 128, 2404–2406. [Google Scholar] [CrossRef] [Green Version]
- Lips, P. Vitamin D status and nutrition in Europe and Asia. J. Steroid. Biochem. Mol. Biol. 2007, 103, 620–625. [Google Scholar] [CrossRef]
- Moy, F.M.; Bulgiba, A. High prevalence of vitamin D insufficiency and its association with obesity and metabolic syndrome among Malay adults in Kuala Lumpur, Malaysia. BMC Public Health. 2011, 11, 735. [Google Scholar] [CrossRef] [Green Version]
- Valcour, A.; Zierold, C.; Podgorski, A.L.; Olson, G.T.; Wall, J.V.; DeLuca, H.F.; Bonelli, F. A novel, fully-automated, chemiluminescent assay for the detection of 1,25-dihydroxyvitamin D in biological samples. J. Steroid. Biochem. Mol. Biol. 2016, 164, 120–126. [Google Scholar] [CrossRef] [Green Version]
- Nurbazlin, M.; Chee, W.S.; Rokiah, P.; Tan, A.T.; Chew, Y.Y.; Nusaibah, A.R.; Chan, S.P. Effects of sun exposure on 25(OH) vitamin D concentration in urban and rural women in Malaysia. Asia Pac. J. Clin. Nutr. 2013, 22, 391–399. [Google Scholar]
- Rahman, S.A.; Chee, W.S.; Yassin, Z.; Chan, SP. Vitamin D status among postmenopausal Malaysian women. Asia Pac. J. Clin. Nutr. 2004, 13, 255–260. [Google Scholar]
- Sng, J.; Koh, D.; Siong, W.C.; Choo, T.B. Skin cancer trends among Asians living in Singapore from 1968 to 2006. J. Am. Acad. Dermatol. 2009, 61, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Tsiaras, W.G.; Weinstock, M.A. Factors influencing vitamin D status. Acta Derm. Venereol. 2011, 91, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, P.; Duan, X.; Wang, J.; Shu, B.; Li, X.; Ba, Q.; Li, J.; Wang, Y.; Wang, H. Bioavailable 25(OH)D but Not Total 25(OH)D Is an Independent Determinant for Bone Mineral Density in Chinese Postmenopausal Women. EBioMedicine 2017, 15, 184–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bischoff-Ferrari, H.A.; Dietrich, T.; Orav, E.J.; Hu, F.B.; Zhang, Y.; Karlson, E.W.; Dawson-Hughes, B. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am. J. Clin. Nutr. 2004, 80, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Heaney, RP. Effects of caffeine on bone and the calcium economy. Food Chem. Toxicol. 2002, 40, 1263–1270. [Google Scholar] [CrossRef]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080s–1086s. [Google Scholar] [CrossRef] [Green Version]
- Johnsen, M.S.; Grimnes, G.; Figenschau, Y.; Torjesen, P.A.; Almås, B.; Jorde, R. Serum free and bio-available 25-hydroxyvitamin D correlate better with bone density than serum total 25-hydroxyvitamin D. Scand. J. Clin. Lab. Investig. 2014, 74, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Aloia, J.F. African Americans, 25-hydroxyvitamin D, and osteoporosis: A paradox. Am. J. Clin. Nutr. 2008, 88, 545s–550s. [Google Scholar] [CrossRef] [Green Version]
- Jemielita, T.O.; Leonard, M.B.; Baker, J.; Sayed, S.; Zemel, B.S.; Shults, J.; Herskovitz, R.; Denburg, M.R. Association of 25-hydroxyvitamin D with areal and volumetric measures of bone mineral density and parathyroid hormone: Impact of vitamin D-binding protein and its assays. Osteoporos. Int. 2016, 27, 617–626. [Google Scholar] [CrossRef] [Green Version]
- Lim, P.S.; Ong, F.B.; Adeeb, N.; Seri, S.S.; Noor-Aini, M.Y.; Shamsuddin, K.; Hapizah, N.; Mohamed, A.L.; Mokhtar, A.; Wan, H.W.H. Bone health in urban midlife Malaysian women: Risk factors and prevention. Osteoporos Int. 2005, 16, 2069–2079. [Google Scholar] [CrossRef]
- Chan, C.Y.; Subramaniam, S.; Mohamed, N.; Ima-Nirwana, S.; Muhammad, N.; Fairus, A.; Ng, P.Y.; Jamil, N.A.; Abd Aziz, N.; Chin, K. Determinants of Bone Health Status in a Multi-Ethnic Population in Klang Valley, Malaysia. Int. J. Environ. Res. Public Health 2020, 17, 384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palermo, A.; Tuccinardi, D.; Defeudis, G.; Watanabe, M.; D’Onofrio, L.; Lauria Pantano, A.; Napoli, N.; Pozzilli, P.; Manfrini, S. BMI and BMD: The Potential Interplay between Obesity and Bone Fragility. Int. J. Environ. Res. Public Health 2016, 13, 544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceglia, L.; Harris, S.S. Vitamin D and its role in skeletal muscle. Calcif. Tissue Int. 2013, 92, 151–162. [Google Scholar] [CrossRef]
- Girgis, C.M.; Clifton-Bligh, R.J.; Hamrick, M.W.; Holick, M.F.; Gunton, J.E. The roles of vitamin D in skeletal muscle: Form, function, and metabolism. Endocr. Rev. 2013, 34, 33–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, M.J.; Yun, S.; Oh, K.; Kim, K. Relation of serum 25-hydroxyvitamin D status with skeletal muscle mass by sex and age group among Korean adults. Br. J. Nutr. 2015, 114, 1838–1844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visser, M.; Deeg, D.J.; Lips, P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): The Longitudinal Aging Study Amsterdam. J. Clin. Endocrinol. Metab. 2003, 88, 5766–5772. [Google Scholar] [CrossRef]
- Abboud, M.; Puglisi, D.A.; Davies, B.N.; Rybchyn, M.; Whitehead, N.P.; Brock, K.E.; Cole, L.; Gordon-Thomson, C.; Fraser, D.R.; Mason, R.S. Evidence for a specific uptake and retention mechanism for 25-hydroxyvitamin D (25OHD) in skeletal muscle cells. Endocrinology 2013, 154, 3022–3030. [Google Scholar] [CrossRef] [Green Version]
- Davis, H.G. Conservative Surgery: As Exhibited in Remedying Some of the Mechanical Causes that Operate Injuriously Both in Health and Disease; Illustrations. D. Appleton: Boston, MA, USA, 1867. [Google Scholar]
- Ahern, T.; Khattak, A.; O’Malley, E.; Dunlevy, C.; Kilbane, M.; Woods, C.; McKenna, M.J.; O’Shea, D. Association Between Vitamin D Status and Physical Function in the Severely Obese. J. Clin. Endocrinol. Metabo. 2014, 99, E1327–E1331. [Google Scholar] [CrossRef] [Green Version]
- Snijder, M.B.; van Schoor, N.M.; Pluijm, S.M.; van Dam, R.M.; Visser, M.; Lips, P. Vitamin D status in relation to one-year risk of recurrent falling in older men and women. J. Clin. Endocrinol. Metab. 2006, 91, 2980–2985. [Google Scholar] [CrossRef] [Green Version]
- Wicherts, I.S.; van Schoor, N.M.; Boeke, A.J.; Visser, M.; Deeg, D.J.; Smit, J.; Knol, D.L.; Lips, P. Vitamin D status predicts physical performance and its decline in older persons. J. Clin. Endocrinol. Metab. 2007, 92, 2058–2065. [Google Scholar] [CrossRef]
- Vermeulen, A.; Verdonck, L.; Kaufman, J.M. A critical evaluation of simple methods for the estimation of free testosterone in serum. J. Clin. Endocrinol. Metabol. 1999, 84, 3666–3672. [Google Scholar] [CrossRef]
- Grampp, S.; Genant, H.K.; Mathur, A.; Lang, P.; Jergas, M.; Takada, M.; Gluer, C.; Lu, Y.; Chavez, M. Comparisons of noninvasive bone mineral measurements in assessing age-related loss, fracture discrimination, and diagnostic classification. J. Bone Miner. Res. 1997, 12, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Fess, E.E. Grip Strength, 2nd ed.; American Society of Hand Therapists: Chicago, IL, USA, 1992. [Google Scholar]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Ji, M.; Song, J.; Moon, H.W.; Hur, M.; Yun, Y.M. Clinical Utility of Measurement of Vitamin D-Binding Protein and Calculation of Bioavailable Vitamin D in Assessment of Vitamin D Status. Ann. Lab. Med. 2017, 37, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Heijboer, A.C.; Blankenstein, M.A.; Kema, I.P.; Buijs, M.M. Accuracy of 6 routine 25-hydroxyvitamin D assays: Influence of vitamin D binding protein concentration. Clin. Chem. 2012, 58, 543–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoaglin, D.C.; Iglewicz, B. Fine-Tuning Some Resistant Rules for Outlier Labeling. J. Am. Stat. Assoc. 1987, 82, 1147–1149. [Google Scholar] [CrossRef]
- Rizzoli, R.; Boonen, S.; Brandi, M.L.; Bruyère, O.; Cooper, C.; Kanis, J.A.; Kaufman, J.; Ringe, J.D.; Weryha, G.; Reginster, J. Vitamin D supplementation in elderly or postmenopausal women: A 2013 update of the 2008 recommendations from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Curr. Med. Res. Opin. 2013, 29, 305–313. [Google Scholar] [CrossRef]
- Rizzoli, R.; Stevenson, J.C.; Bauer, J.M.; van Loon, L.J.; Walrand, S.; Kanis, J.A.; Cooper, C.; Brandi, M.; Diez-Perez, A.; Reginster, J.; et al. The role of dietary protein and vitamin D in maintaining musculoskeletal health in postmenopausal women: A consensus statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Maturitas 2014, 79, 122–132. [Google Scholar] [CrossRef]
- Nagaraj, S.; Nai-Peng, T.; Chiu-Wan, N.; Kiong-Hock, L.; Pala, J. Counting ethnicity in Malaysia: The complexity of measuring diversity. In Social Statistics and Ethnic Diversity; Springer: Cham, Switzerland, 2015; pp. 143–173. [Google Scholar]
- Bouillon, R.; Schuit, F.; Antonio, L.; Rastinejad, F. Vitamin D Binding Protein: A Historic Overview. Front. Endocrinol. 2020, 10, 910. [Google Scholar] [CrossRef]


| Variables | Reference | N | Mean ± SD/Median (IQR) | Minimum–Maximum | |
|---|---|---|---|---|---|
| Age (years) | 141 | 59.0(10.0) | 45.0–88.0 | ||
| Age at menopause (years) | 121 | 50.0(6.0) | 36.0–59.0 | ||
| Years since menopause | 121 | 8.0(11.0) | 1.0–35.0 | ||
| Height (cm) | 141 | 153.1 ± 6.2 | 137.5–169.0 | ||
| Weight (kg) | 141 | 63.4 ± 12.6 | 31.9–100.9 | ||
| BMI (kg/m2) δ | All | 141 | 27.1 ± 5.3 | 15.4–43.0 | |
| Normal | 18.5–22.99 | 29 | 20.8 ± 1.3 | 18.5–22.8 | |
| Overweight | 23.0–27.49 | 40 | 25.0(2.65) | 23.1–27.4 | |
| Obese Type 1 and 2 | ≥27.5 | 68 | 30.25(4.1) | 27.5–43.0 | |
| Waist circ. (cm) µ | All | 139 | 84.2 ± 12.6 | 55.1–121.0 | |
| Overweight/Obese | ≥80 cm | 87 | 89.7(11.2) | 80.0–121.0 | |
| Body fat (%) | All | 141 | 41.8(10.45) | 20.7–54.0 | |
| Obese γ | ≥32% | 121 | 43.1(8.8) | 32.3–54.0 | |
| AppSMMI (kg/m2) | All | 140 | 6.1(1.1) | 4.0–10.7 | |
| Sarcopenic β | ≤5.7 kg/m2 | 44 | 5.3(0.4) | 4.01–5.69 | |
| BUA (dB/MHz) | All | 139 | 70.0 ± 16.8 | 35.9–122.2 | |
| Osteopenic α | <54 dB/MHz | 25 | 47.5(9.75) | 35.9–53.8 | |
| Total 25(OH)D (nmol/L) ƒ | >50 nmol/L | 120 | 51.0(23.85) | 23.0–117.6 | |
| Serum Calcium (mmol/L) ƚ | 2.10–2.55 mmol/L | 120 | 2.4 ± 0.1 | 2.15–2.65 | |
| Plasma iPTH (pmol/L) ƚ | 1.5–7.6 pmol/L | 119 | 5.2(3.7) | 1.60–12.30 | |
| Serum albumin (g/L) ƚ | 35–50 g/L | 120 | 45.0(5.0) | 38.0–50.0 | |
| Bioavailable 25(OH)D (nmol/L) | 116 | 6.2(3.2) | 2.83–17.0 | ||
| Bioavailable 25(OH)D (ng/mL) ƚ | 1.92–8.82 ng/mL | 116 | 2.5 (1.3) | 1.13–6.80 | |
| Free 25(OH)D (pmol/L) | 116 | 15.7(7.4) | 6.87–43.2 | ||
| VDBP (ug/mL) ƚ | 104–477 ug/mL | 116 | 224.7 ± 44.8 | 123.5–327.7 |
| Variables | N Total | Breakdown of variables | n | Percentage (%) |
|---|---|---|---|---|
| Education Levels | 140 | No Formal Education | 10 | 7.1 |
| Primary School | 22 | 15.7 | ||
| Secondary School | 55 | 39.3 | ||
| Certificate/Diploma | 29 | 20.7 | ||
| University Degree | 19 | 13.6 | ||
| Postgraduate Degree | 5 | 3.6 | ||
| Cigarette Smoking Status | 139 | Non-smoker | 137 | 98.6 |
| Current smoker | 2 | 1.4 | ||
| Alcohol Drinking | 138 | Non-drinker | 136 | 97.8 |
| Current drinker | 2 | 2.2 | ||
| Self-rated PA Status | 137 | Inactive | 69 | 50.4 |
| Active (at least 10 mins per day) | 68 | 49.6 | ||
| Disease(s)/disorder(s) | 139 | None | 60 | 43.2 |
| Hypertension | 53 | |||
| Diabetes Type 2 | 31 | |||
| Heart problems | 11 | |||
| Osteoarthritis | 12 | |||
| Rheumatoid Arthritis | 7 | |||
| Osteoporosis | 8 | |||
| Have had stroke | 4 | |||
| Depression/anxiety | 6 |
| Variables | Deficient (<30 nmol/L)ƒ Mean (SD), n = 9 | Insufficient (30–50 nmol/L)ƒ Mean (SD), n = 49 | Replete (>50 nmol/L)ƒ Mean (SD), n = 62 | p-Value * |
|---|---|---|---|---|
| HGS (kg) | 20.7 (5.5) | 18.7 (4.7) | 20.8 (4.6) | 0.091 |
| AppSMMI (kg/m2) | 6.0 (1.3) | 6.1 (0.8) | 6.1 (0.7) | 0.978 |
| SMMI (kg) | 8.7 (1.5) | 8.4 (1.0) | 8.4 (0.9) | 0.822 |
| FFMI (kg) | 16.3 (2.3) | 15.8 (1.6) | 15.7 (1.5) | 0.695 |
| BFP (%) | 43.6 (9.8) ** | 43.1 (6.7) ** | 38.8 (8.1) | 0.020 |
| BMI (kg/m2) | 29.9 (7.5) | 28.3 (5.5) | 26.1 (4.9) | 0.073 |
| WC (cm) | 89.0 (14.8) | 84.6 (12.6) | 83.9 (12.4) | 0.625 |
| BUA (dB/MHz) | 56.5 (16.6) ** | 68.5 (15.8) | 72.2 (17.4) | 0.047 |
| Sit-to-stand test (times in 30 s) | 11.8 (2.6) | 11.1 (3.7) | 12.4 (3.6) | 0.213 |
| Walk speed (m/s) | 0.7 (0.2) ** | 0.9 (0.2) ** | 1.0 (0.3) | 0.010 |
| Balance (sec) | 17.9 (11.7) | 17.3 (11.8) | 21.3 (9.8) | 0.200 |
| Calcium (mmol/L) | 2.3 (0.1) ** | 2.4 (0.1) | 2.4 (0.1) | 0.015 |
| iPTH (pmol/L) | 9.1 (3.1) ** | 6.7 (2.5) ** | 4.7 (2.2) | 0.000 |
| Bioavailable 25(OH)D (nmol/L) | 3.6 (0.6) **# | 5.3 (1.2) ** | 8.8 (3.0) | 0.000 |
| Free 25(OH)D (pmol/L) | 9.2 (1.6) **# | 13.2 (3.1) ** | 22.0 (7.0) | 0.000 |
| VDBP (ug/mL) | 206.1 (36.8) | 227.1 (48.3) | 225.6 (43.0) | 0.328 |
| Parameters | Total 25(OH)D (nmol/L) | Bio 25(OH)D (nmol/L) | VDBP (ug/mL) | Calcium (mmol/L) | iPTH (pmol/L) | BFP (%) | BUA (dB/MHz) | AppSMMI (kg/m2) | STS | GS (m/s) |
|---|---|---|---|---|---|---|---|---|---|---|
| Total 25(OH)D | 1 | 0.883 ** | −0.045 | 0.192* | −0.394 ** | −0.298 ** | 0.199 * | 0.012 | 0.129 | 0.191 * |
| Bio 25(OH)D | 0.883 ** | 1 | −0.446** | 0.238* | −0.426 ** | −0.380 ** | 0.234 * | −0.007 | 0.202 * | 0.134 |
| VDBP | −0.045 | −0.446 ** | 1 | 0.123 | 0.071 | 0.149 | −0.080 | 0.124 | 0.002 | −0.115 |
| Calcium | 0.192 * | 0.238 * | 0.123 | 1 | −0.497 ** | −0.294 ** | 0.072 | −0.170 | 0.164 | 0.045 |
| iPTH | −0.394 ** | −0.426 ** | 0.071 | −0.497** | 1 | 0.448 ** | −0.117 | 0.241 ** | −0.249 ** | −0.067 |
| BFP | −0.298 ** | −0.380 ** | 0.149 | −0.294** | 0.448 ** | 1 | 0.043 | 0.359 ** | −0.198 * | −0.046 |
| BUA | 0.199* | 0.234 * | −0.080 | 0.072 | −0.117 | 0.043 | 1 | 0.192 * | 0.143 | 0.050 |
| AppSMMI | 0.012 | −0.007 | 0.124 | −0.170 | 0.241 ** | 0.359 ** | 0.192 * | 1 | 0.011 | 0.248 ** |
| STS | 0.129 | 0.202 * | 0.002 | 0.164 | −0.249** | −0.198 * | 0.143 | 0.011 | 1 | 0.350 ** |
| GS | 0.191 * | 0.134 | −0.115 | 0.045 | −0.067 | −0.046 | 0.050 | 0.248 ** | 0.350 ** | 1 |
| Dependent Variables | Predictors | Significant Predictors | Beta | t Value | Significance | R2 | Adjusted R2 | F |
|---|---|---|---|---|---|---|---|---|
| BUA (dB/MHz) | Age (years) | Age | −0.191 | −1.939 | 0.055 * | 0.090 | 0.072 | 4.774 ** |
| Years since menopause | Bio 25(OH)D | 0.267 | 2.700 | 0.008 *** | ||||
| BMI (kg/m2) | ||||||||
| BFP (%) | ||||||||
| Total 25(OH)D (nmol/L) | ||||||||
| Bio 25(OH)D (nmol/L) | ||||||||
| Free 25(OH)D (pmol/L) | ||||||||
| AppSMMI (kg/m2) | Age (years) | BMI | 1.326 | 11.665 | 0.000 *** | 0.650 | 0.639 | 58.821 *** |
| Years since menopause | BFP | −0.716 | −6.112 | 0.000 *** | ||||
| BMI (kg/m2) | Bio 25(OH)D | 0.120 | 1.824 | 0.071 * | ||||
| BFP (%) | ||||||||
| Total 25(OH)D (nmol/L) | ||||||||
| Bio 25(OH)D (nmol/L) | ||||||||
| Free 25(OH)D (pmol/L) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abidin, N.Z.; Mitra, S.R. Total vs. Bioavailable: Determining a Better 25(OH)D Index in Association with Bone Density and Muscle Mass in Postmenopausal Women. Metabolites 2021, 11, 23. https://doi.org/10.3390/metabo11010023
Abidin NZ, Mitra SR. Total vs. Bioavailable: Determining a Better 25(OH)D Index in Association with Bone Density and Muscle Mass in Postmenopausal Women. Metabolites. 2021; 11(1):23. https://doi.org/10.3390/metabo11010023
Chicago/Turabian StyleAbidin, Nurdiana Z., and Soma R. Mitra. 2021. "Total vs. Bioavailable: Determining a Better 25(OH)D Index in Association with Bone Density and Muscle Mass in Postmenopausal Women" Metabolites 11, no. 1: 23. https://doi.org/10.3390/metabo11010023
APA StyleAbidin, N. Z., & Mitra, S. R. (2021). Total vs. Bioavailable: Determining a Better 25(OH)D Index in Association with Bone Density and Muscle Mass in Postmenopausal Women. Metabolites, 11(1), 23. https://doi.org/10.3390/metabo11010023