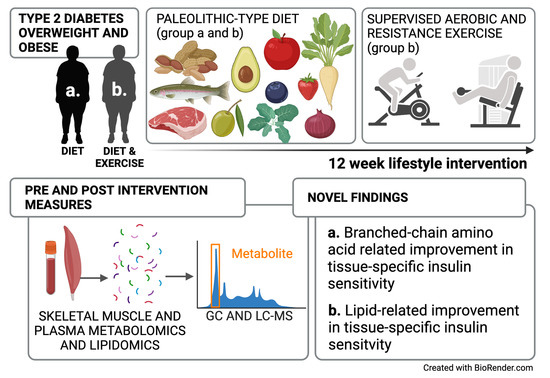
Improved Peripheral and Hepatic Insulin Sensitivity after Lifestyle Interventions in Type 2 Diabetes Is Associated with Specific Metabolomic and Lipidomic Signatures in Skeletal Muscle and Plasma
Abstract
:1. Introduction
2. Results
2.1. Clinical Data
2.2. Metabolomics and Lipidomics
2.2.1. Skeletal Muscle Metabolomics and Lipidomics
2.2.2. Plasma Metabolomics and Lipidomics
2.3. Intervention Response in Skeletal Muscle Metabolites and Lipids That Describe Improved Tissue-Specific Insulin Sensitivity
2.4. Intervention Response in Circulating Metabolites and Lipids That Describe Improved Tissue-Specific Insulin Senstitivty
3. Discussion
4. Materials and Methods
4.1. Research Design and Sampling Procedure
4.2. Body Composition, Tissue-Specific Insulin Sensitivity, Liver and Soleus Fat, Vo2max, and Fat Oxidation Capacity
4.3. Mass Spectrometry-Based Metabolomics Analysis
4.3.1. Sample Preparation
4.3.2. Data Processing
4.3.3. Statistical Analysis and Bioinformatics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IDF. Available online: https://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html (accessed on 11 October 2021).
- Panunzi, S.; Carlsson, L.; De Gaetano, A.; Peltonen, M.; Rice, T.; Sjöström, L.; Mingrone, G.; Dixon, J.B. Determinants of Diabetes Remission and Glycemic Control After Bariatric Surgery. Diabetes Care 2015, 39, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R.; Al-Mrabeh, A.; Zhyzhneuskaya, S.; Peters, C.; Barnes, A.C.; Aribisala, B.S.; Hollingsworth, K.G.; Mathers, J.C.; Sattar, N.; Lean, M.E. Remission of Human Type 2 Diabetes Requires Decrease in Liver and Pancreas Fat Content but Is Dependent upon Capacity for β Cell Recovery. Cell Metab. 2018, 28, 547–556.e3. [Google Scholar] [CrossRef] [Green Version]
- Al-Mrabeh, A.; Zhyzhneuskaya, S.V.; Peters, C.; Barnes, A.C.; Melhem, S.; Jesuthasan, A.; Aribisala, B.; Hollingsworth, K.G.; Lietz, G.; Mathers, J.C.; et al. Hepatic Lipoprotein Export and Remission of Human Type 2 Diabetes after Weight Loss. Cell Metab. 2020, 31, 233–249.e4. [Google Scholar] [CrossRef]
- Perreault, L.; Newsom, S.A.; Strauss, A.; Kerege, A.; Kahn, D.E.; Harrison, K.A.; Snell-Bergeon, J.K.; Nemkov, T.; D’Alessandro, A.; Jackman, M.R.; et al. Intracellular localization of diacylglycerols and sphingolipids influences insulin sensitivity and mitochondrial function in human skeletal muscle. JCI Insight 2018, 3, e96805. [Google Scholar] [CrossRef] [Green Version]
- Goodpaster, B.H.; He, J.; Watkins, S.; Kelley, D.E. Skeletal Muscle Lipid Content and Insulin Resistance: Evidence for a Paradox in Endurance-Trained Athletes. J. Clin. Endocrinol. Metab. 2001, 86, 5755–5761. [Google Scholar] [CrossRef]
- Ohashi, K.; Fujii, M.; Uda, S.; Kubota, H.; Komada, H.; Sakaguchi, K.; Ogawa, W.; Kuroda, S. Increase in hepatic and decrease in peripheral insulin clearance characterize abnormal temporal patterns of serum insulin in diabetic subjects. NPJ Syst. Biol. Appl. 2018, 4, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- She, P.; Van Horn, C.; Reid, T.; Hutson, S.M.; Cooney, R.N.; Lynch, C.J. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am. J. Physiol. Metab. 2007, 293, E1552–E1563. [Google Scholar] [CrossRef] [Green Version]
- Newgard, C.B. Interplay between Lipids and Branched-Chain Amino Acids in Development of Insulin Resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef] [Green Version]
- White, P.J.; Lapworth, A.L.; An, J.; Wang, L.; McGarrah, R.W.; Stevens, R.D.; Ilkayeva, O.; George, T.; Muehlbauer, M.J.; Bain, J.R.; et al. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol. Metab. 2016, 5, 538–551. [Google Scholar] [CrossRef] [PubMed]
- Otten, J.; Stomby, A.; Waling, M.; Isaksson, A.; Tellström, A.; Lundin-Olsson, L.; Brage, S.; Ryberg, M.; Svensson, M.; Olsson, T. Benefits of a Paleolithic diet with and without supervised exercise on fat mass, insulin sensitivity, and glycemic control: A randomized controlled trial in individuals with type 2 diabetes. Diabetes/Metab. Res. Rev. 2017, 33, e2828. [Google Scholar] [CrossRef]
- Ibrahim, N.; Fairus, S.; Zulfarina, M.S.; Naina Mohamed, I. The Efficacy of Squalene in Cardiovascular Disease Risk-A Systematic Review. Nutrients 2020, 12, 414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walters, R.O.; Arias, E.; Diaz, A.; Burgos, E.S.; Guan, F.; Tiano, S.; Mao, K.; Green, C.L.; Qiu, Y.; Shah, H.; et al. Sarcosine Is Uniquely Modulated by Aging and Dietary Restriction in Rodents and Humans. Cell Rep. 2018, 25, 663–676.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, S.; Nielsen, J.; Hansen, C.N.; Nielsen, L.B.; Wibrand, F.; Stride, N.; Schrøder, H.D.; Boushel, R.; Helge, J.W.; Dela, F.; et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J. Physiol. 2012, 590, 3349–3360. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.; Oh, S.F.; Wada, S.; Rowe, G.; Liu, L.; Chan, M.C.; Rhee, J.; Hoshino, A.; Kim, B.; Ibrahim, A.; et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat. Med. 2016, 22, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.; Yoo, H.J.; Kim, M.; Kim, M.; Lee, J.H. Metabolomics identifies increases in the acylcarnitine profiles in the plasma of overweight subjects in response to mild weight loss: A randomized, controlled design study. Lipids Health Dis. 2018, 17, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendham, A.E.; Goedecke, J.H.; Zeng, Y.; Larsen, S.; George, C.; Hauksson, J.; Smidt, M.C.F.-D.; Chibalin, A.V.; Olsson, T.; Chorell, E. Exercise training improves mitochondrial respiration and is associated with an altered intramuscular phospholipid signature in women with obesity. Diabetologia 2021, 64, 1642–1659. [Google Scholar] [CrossRef]
- White, P.J.; Lapworth, A.L.; McGarrah, R.W.; Kwee, L.C.; Crown, S.B.; Ilkayeva, O.; An, J.; Carson, M.W.; Christopher, B.A.; Ball, J.R.; et al. Muscle-Liver Trafficking of BCAA-Derived Nitrogen Underlies Obesity-Related Glycine Depletion. Cell Rep. 2020, 33, 108375. [Google Scholar] [CrossRef] [PubMed]
- Shulman, G.I. Ectopic Fat in Insulin Resistance, Dyslipidemia, and Cardiometabolic Disease. N. Engl. J. Med. 2014, 371, 1131–1141. [Google Scholar] [CrossRef]
- Letto, J.; E Brosnan, M.; Brosnan, J.T. Valine metabolism Gluconeogenesis from 3-hydroxyisobutyrate. Biochem. J. 1986, 240, 909–912. [Google Scholar] [CrossRef] [Green Version]
- Daemen, S.; Gemmink, A.; Brouwers, B.; Meex, R.C.; Huntjens, P.R.; Schaart, G.; Moonen-Kornips, E.; Jörgensen, J.; Hoeks, J.; Schrauwen, P.; et al. Distinct lipid droplet characteristics and distribution unmask the apparent contradiction of the athlete’s paradox. Mol. Metab. 2018, 17, 71–81. [Google Scholar] [CrossRef]
- Lerin, C.; Goldfine, A.B.; Boes, T.; Liu, M.; Kasif, S.; Dreyfuss, J.; De Sousa-Coelho, A.L.; Daher, G.; Manoli, I.; Sysol, J.R.; et al. Defects in muscle branched-chain amino acid oxidation contribute to impaired lipid metabolism. Mol. Metab. 2016, 5, 926–936. [Google Scholar] [CrossRef]
- Shi, H.; Munk, A.; Nielsen, T.S.; Daughtry, M.R.; Larsson, L.; Li, S.; Høyer, K.F.; Geisler, H.W.; Sulek, K.; Kjøbsted, R.; et al. Skeletal muscle O-GlcNAc transferase is important for muscle energy homeostasis and whole-body insulin sensitivity. Mol. Metab. 2018, 11, 160–177. [Google Scholar] [CrossRef]
- Meikle, P.; Summers, S. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat. Rev. Endocrinol. 2017, 13, 79–91. [Google Scholar] [CrossRef]
- Bailey, A.; Koster, G.; Guillermier, C.; Hirst, E.M.; MacRae, J.I.; Lechene, C.P.; Postle, A.; Gould, A.P. Antioxidant Role for Lipid Droplets in a Stem Cell Niche of Drosophila. Cell 2015, 163, 340–353. [Google Scholar] [CrossRef] [Green Version]
- Mårtensson, A.; Stomby, A.; Tellström, A.; Ryberg, M.; Waling, M.; Otten, J. Using a Paleo Ratio to Assess Adherence to Paleolithic Dietary Recommendations in a Randomized Controlled Trial of Individuals with Type 2 Diabetes. Nutrients 2021, 13, 969. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, M.; Chiefari, E.; Arcidiacono, B.; Corigliano, D.M.; Brunetti, F.S.; Maggisano, V.; Russo, D.; Foti, D.P.; Brunetti, A. Mediterranean Diet Nutrients to Turn the Tide against Insulin Resistance and Related Diseases. Nutrients 2020, 12, 1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otten, J.; Stomby, A.; Waling, M.; Isaksson, A.; Söderström, I.; Ryberg, M.; Svensson, M.; Hauksson, J.; Olsson, T. A heterogeneous response of liver and skeletal muscle fat to the combination of a Paleolithic diet and exercise in obese individuals with type 2 diabetes: A randomised controlled trial. Diabetol. 2018, 61, 1548–1559. [Google Scholar] [CrossRef] [Green Version]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults: Guidance for Prescribing Exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Aldenbäck, E.; Johansson, H.-E. Anthropometric Measurements and Correlations to Glucometabolic and Cardiovascular Risk in Obese Patients Undergoing Gastric Bypass Surgery. J. Obes. 2021, 2021, 6647328. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E.; Wallis, G.A. Measurement of Substrate Oxidation during Exercise by Means of Gas Exchange Measurements. Int. J. Sports Med. 2004, 26, S28–S37. [Google Scholar] [CrossRef]
- Jonsson, P.; Wuolikainen, A.; Thysell, E.; Chorell, E.; Stattin, P.; Wikström, P.; Antti, H. Constrained randomization and multivariate effect projections improve information extraction and biomarker pattern discovery in metabolomics studies involving dependent samples. Metabolomics 2015, 11, 1667–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trygg, J.; Gullberg, J.; Johansson, A.I.; Jonsson, P.; Antti, H.; Marklund, S.L.; Moritz, T. Extraction and GC/MS Analysis of the Human Blood Plasma Metabolome. Anal. Chem. 2005, 77, 8086–8094. [Google Scholar] [CrossRef]
- Nygren, H.; Seppänen-Laakso, T.; Castillo, S.; Hyötyläinen, T.; Orešič, M. Liquid Chromatography-Mass Spectrometry (LC-MS)-Based Lipidomics for Studies of Body Fluids and Tissues. In Protein Microarray for Disease Analysis; Springer: Singapore, 2010; Volume 708, pp. 247–257. [Google Scholar]
- Gullberg, J.; Jonsson, P.; Nordström, A.; Sjöström, M.; Moritz, T. Design of experiments: An efficient strategy to identify factors influencing extraction and derivatization of Arabidopsis thaliana samples in metabolomic studies with gas chromatography/mass spectrometry. Anal. Biochem. 2004, 331, 283–295. [Google Scholar] [CrossRef]
- Efron, B.; Gong, G. A Leisurely Look at the Bootstrap, the Jackknife, and Cross-Validation; Taylor & Francis, Ltd.: Hoboken, NJ, USA, 1983; Volume 37. [Google Scholar]
- Chorell, E.; Ryberg, M.; Larsson, C.; Sandberg, S.; Mellberg, C.; Lindahl, B.; Antti, H.; Olsson, T. Plasma metabolomic response to postmenopausal weight loss induced by different diets. Metabolomics 2016, 12, 85. [Google Scholar] [CrossRef]





| Diet Group | Diet-Exercise Group | |||
|---|---|---|---|---|
| PRE (Week 0) | POST (Week 12) | PRE (Week 0) | POST (Week 12) | |
| Female subjects (male subject), n | 12 (9) | 14 (9) | ||
| Age, years | 58.3 ± 6.8 | 62.3 ± 4.5 | ||
| Diabetes duration, years | 4.2 ± 3.2 | 4.5 ± 3.4 | ||
| Weight, kg | 94.5 ± 14.2 | 86.0 ± 11.8 * | 95.5 ± 17.5 | 89.0 ± 17.1 * |
| BMI, kg/m2 | 31.6 ± 3.0 | 28.7 ± 2.2 * | 31.9 ± 3.7 | 29.6 ± 3.9 * |
| Sagittal abdominal diameter, cm | 27.1 ± 3.4 | 23.2 ± 2.9 * | 26.3 ± 3.7 | 23.7 ± 3.2 * |
| VO2 peak, ml/min | 2303.6 ± 362.2 | 2265.3 ± 351.1 | 2270.5 ± 647.9 | 2428.0 ± 593.2 * |
| Waist circumference, cm | 110.5 ± 9.8 | 100.6 ± 8.1 * | 109.7 ± 10.9 | 102.5 ± 12.6 * |
| Lean mass | 58.1 ± 10.9 | 55.5 ± 9.2 * | 56.9 ± 13.0 | 57.0 ± 12.9 |
| Fat mass, % | 36.2 ± 6.1 | 26.8 ± 6.7 * | 38.3 ± 5.0 | 33.5 ± 5.7 * |
| CRP, nmol/l | 1.7 ± 1.3 | 1.3 ± 0.9 | 1.8 ± 1.3 | 1.2 ± 0.8 * |
| HbA1c, % | 6.2 ± 1.7 | 5.2 ± 1.7 * | 6.7 ± 2.4 | 5.3 ± 2.3 * |
| INSULIN SENSITIVITY | PRE (Week 0) | POST (Week 12) | PRE (Week 0) | POST (Week 12) |
| Hepatic insulin sensitivity a | 1.1 ± 0.5 | 1.5 ± 0.4 * | 1.3 ± 0.5 | 1.3 ± 0.5 |
| Peripheral insulin sensitivity b | 0.04 ± 0.02 | 0.08 ± 0.04 * | 0.05 ± 0.02 | 0.07 ± 0.02 * |
| Insulin resistance estimate (HOMA-IR) c | 7.5 ± 2.4 | 4.0 ± 1.9 * | 7.2 ± 3.0 | 3.6 ± 2.0 * |
| ECTOPIC FAT | PRE (Week 0) | POST (Week 12) | PRE (Week 0) | POST (Week 12) |
| Liver fat, % | 18.1 ± 9.7 | 6.1 ± 5.9 * | 15.3 ± 9.8 | 9.7 ± 8.3 |
| Skeletal muscle fat, % in soleus | 20.4 ± 8.5 | 11.9 ± 6.4 * | 22.8 ± 14.8 | 19.8 ± 16.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chorell, E.; Otten, J.; Stomby, A.; Ryberg, M.; Waling, M.; Hauksson, J.; Svensson, M.; Olsson, T. Improved Peripheral and Hepatic Insulin Sensitivity after Lifestyle Interventions in Type 2 Diabetes Is Associated with Specific Metabolomic and Lipidomic Signatures in Skeletal Muscle and Plasma. Metabolites 2021, 11, 834. https://doi.org/10.3390/metabo11120834
Chorell E, Otten J, Stomby A, Ryberg M, Waling M, Hauksson J, Svensson M, Olsson T. Improved Peripheral and Hepatic Insulin Sensitivity after Lifestyle Interventions in Type 2 Diabetes Is Associated with Specific Metabolomic and Lipidomic Signatures in Skeletal Muscle and Plasma. Metabolites. 2021; 11(12):834. https://doi.org/10.3390/metabo11120834
Chicago/Turabian StyleChorell, Elin, Julia Otten, Andreas Stomby, Mats Ryberg, Maria Waling, Jon Hauksson, Michael Svensson, and Tommy Olsson. 2021. "Improved Peripheral and Hepatic Insulin Sensitivity after Lifestyle Interventions in Type 2 Diabetes Is Associated with Specific Metabolomic and Lipidomic Signatures in Skeletal Muscle and Plasma" Metabolites 11, no. 12: 834. https://doi.org/10.3390/metabo11120834
APA StyleChorell, E., Otten, J., Stomby, A., Ryberg, M., Waling, M., Hauksson, J., Svensson, M., & Olsson, T. (2021). Improved Peripheral and Hepatic Insulin Sensitivity after Lifestyle Interventions in Type 2 Diabetes Is Associated with Specific Metabolomic and Lipidomic Signatures in Skeletal Muscle and Plasma. Metabolites, 11(12), 834. https://doi.org/10.3390/metabo11120834