Mass Spectrometry-Based Flavor Monitoring of Peruvian Chocolate Fabrication Process
Abstract
:1. Introduction
2. Results
2.1. Identification of Volatile Compounds from Northern Peruvian Chocolate
2.2. Flavor VOCs from Northern Peru
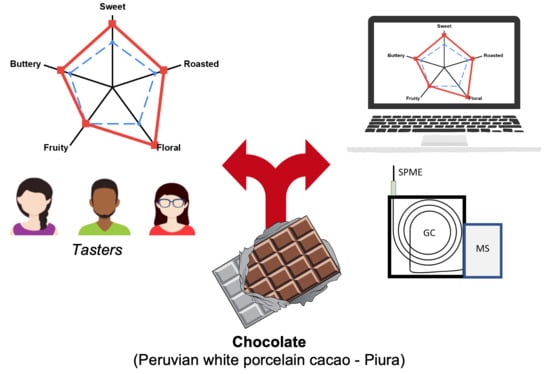
2.3. Taster’s Results vs. MS
2.4. Flavor Development during Each Key Step of Chocolate Elaboration
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sample Preparation and Volatile Compounds Extraction
4.3. HS-SPME-GC-MS Method
4.4. Sensory Analysis
4.5. Selection of Five Groups of Flavors
4.6. Experimental Design and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santander Muñoz, M.; Rodríguez Cortina, J.; Vaillant, F.E.; Escobar Parra, S. An Overview of the Physical and Biochemical Transformation of Cocoa Seeds to Beans and to Chocolate: Flavor Formation. Crit. Rev. Food Sci. Nutr. 2020, 60, 1593–1613. [Google Scholar] [CrossRef] [PubMed]
- Aprotosoaie, A.C.; Luca, S.V.; Miron, A. Flavor Chemistry of Cocoa and Cocoa Products-An Overview: Flavor Chemistry of Cocoa. Compr. Rev. Food Sci. Food Saf. 2016, 15, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Cambrai, A.; Marcic, C.; Morville, S.; Sae Houer, P.; Bindler, F.; Marchioni, E. Differentiation of Chocolates According to the Cocoa’s Geographical Origin Using Chemometrics. J. Agric. Food Chem. 2010, 58, 1478–1483. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Ryan, A. Flavor Formation and Character in Cocoa and Chocolate: A Critical Review. Crit. Rev. Food Sci. Nutr. 2008, 48, 840–857. [Google Scholar] [CrossRef]
- Hoskin, J.C.; Dimick, P.S. Chemistry of Flavour Development in Chocolate. In Industrial Chocolate Manufacture and Use; Beckett, S.T., Ed.; Springer: Boston, MA, USA, 1994. [Google Scholar]
- Counet, C.; Callemien, D.; Ouwerx, C.; Collin, S. Use of Gas Chromatography—Olfactometry To Identify Key Odorant Compounds in Dark Chocolate. Comparison of Samples before and after Conching. J. Agric. Food Chem. 2002, 50, 2385–2391. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.; Lieberei, R. Biochemistry of Cocoa Fermentation. In Cocoa and Coffee Fermentations; CRC Press: Boca Raton, FL, USA, 2015; pp. 193–226. [Google Scholar]
- Caligiani, A.; Marseglia, A.; Prandi, B.; Palla, G.; Sforza, S. Influence of Fermentation Level and Geographical Origin on Cocoa Bean Oligopeptide Pattern. Food Chem. 2016, 211, 431–439. [Google Scholar] [CrossRef]
- Marseglia, A.; Musci, M.; Rinaldi, M.; Palla, G.; Caligiani, A. Volatile Fingerprint of Unroasted and Roasted Cocoa Beans (Theobroma Cacao, L.) from Different Geographical Origins. Food Res. Int. 2020, 132, 109101. [Google Scholar] [CrossRef]
- Jahurul, M.H.A.; Zaidul, I.S.M.; Norulaini, N.A.N.; Sahena, F.; Jinap, S.; Azmir, J.; Sharif, K.M.; Omar, A.K.M. Cocoa Butter Fats and Possibilities of Substitution in Food Products Concerning Cocoa Varieties, Alternative Sources, Extraction Methods, Composition, and Characteristics. J. Food Eng. 2013, 117, 467–476. [Google Scholar] [CrossRef]
- Fine or Flavour Cocoa. Available online: www.icco:about-cocoa/fine-or-flavour-cocoa.html (accessed on 2 October 2020).
- Counet, C.; Ouwerx, C.; Rosoux, D.; Collin, S. Relationship between Procyanidin and Flavor Contents of Cocoa Liquors from Different Origins. J. Agric. Food Chem. 2004, 52, 6243–6249. [Google Scholar] [CrossRef]
- Elwers, S.; Zambrano, A.; Rohsius, C.; Lieberei, R. Differences between the Content of Phenolic Compounds in Criollo, Forastero and Trinitario Cocoa Seed (Theobroma Cacao, L.). Eur. Food Res. Technol. 2009, 229, 937–948. [Google Scholar] [CrossRef]
- Crafack, M.; Keul, H.; Eskildsen, C.E.; Petersen, M.A.; Skovmand-Larsen, M.; Swiegers, J.H.; Petersen, G.B.; Heimdal, H.; Nielsen, D.S. Impact of Starter Cultures and Fermentation Techniques on the Volatile Aroma and Sensory Profile of Chocolate. Food Res. Int. 2014, 63, 306–316. [Google Scholar] [CrossRef]
- Bastos, V.S.; Uekane, T.M.; Bello, N.A.; de Rezende, C.M.; Flosi Paschoalin, V.M.; del Aguila, E.M. Dynamics of Volatile Compounds in TSH 565 Cocoa Clone Fermentation and Their Role on Chocolate Flavor in Southeast Brazil. J. Food Sci. Technol. 2019, 56, 2874–2887. [Google Scholar] [CrossRef]
- Da Veiga Moreira, I.M.; de Figueiredo Vilela, L.; Santos, C.; Lima, N.; Schwan, R.F. Volatile Compounds and Protein Profiles Analyses of Fermented Cocoa Beans and Chocolates from Different Hybrids Cultivated in Brazil. Food Res. Int. 2018, 109, 196–203. [Google Scholar] [CrossRef]
- Oberrauter, L.-M.; Januszewska, R.; Schlich, P.; Majchrzak, D. Sensory Evaluation of Dark Origin and Non-Origin Chocolates Applying Temporal Dominance of Sensations (TDS). Food Res. Int. 2018, 111, 39–49. [Google Scholar] [CrossRef]
- Thomas, E.; van Zonneveld, M.; Loo, J.; Hodgkin, T.; Galluzzi, G.; van Etten, J. Present Spatial Diversity Patterns of Theobroma Cacao, L. in the Neotropics Reflect Genetic Differentiation in Pleistocene Refugia Followed by Human-Influenced Dispersal. PLoS ONE 2012, 7, 47676. [Google Scholar] [CrossRef] [Green Version]
- Bartley, G. The Genetic Diversity of Cacao and Its Utilization; CABI Publishing: Wallingford, UK, 2005. [Google Scholar]
- Rojas, R.; Rodríguez, C.; Ruiz, C.; Portales, R.; Neyra, E.; Patel, K.; Mogrovejo, J.; Salazar, G.; Hurtado, J. Cacao Chuncho Del Cuzco; Universidad Peruana Cayetano Heredia: Lima, Peru, 2017. [Google Scholar]
- Arévalo-Gardini, E.; Arévalo-Hernández, C.O.; Baligar, V.C.; He, Z.L. Heavy Metal Accumulation in Leaves and Beans of Cacao (Theobroma Cacao, L.) in Major Cacao Growing Regions in Peru. Sci. Total Environ. 2017, 605, 792–800. [Google Scholar] [CrossRef]
- Machado Cuellar, L.; Ordoñez Espinosa, C.M.; Angel Sanchez, Y.K.; Guaca Cruz, L.; Suárez Salazar, J.C. Organoleptic Quality Assessment of Theobroma Cacao, L. in Cocoa Farms in Northern Huila, Colombia. Acta Agron. 2018, 67, 46–52. [Google Scholar] [CrossRef]
- Fayeulle, N.; Preys, S.; Roger, J.-M.; Boulanger, R.; Hue, C.; Cheynier, V.; Sommerer, N. Multiblock Analysis to Relate Polyphenol Targeted Mass Spectrometry and Sensory Properties of Chocolates and Cocoa Beans. Metabolites 2020, 10, 311. [Google Scholar] [CrossRef]
- Langenau, J.; Oluwagbemigun, K.; Brachem, C.; Lieb, W.; Giuseppe, R.D.; Artati, A.; Kastenmüller, G.; Weinhold, L.; Schmid, M.; Nöthlings, U. Blood Metabolomic Profiling Confirms and Identifies Biomarkers of Food Intake. Metabolites 2020, 10, 468. [Google Scholar] [CrossRef]
- Deuscher, Z.; Gourrat, K.; Repoux, M.; Boulanger, R.; Labouré, H.; Le Quéré, J.-L. Key Aroma Compounds of Dark Chocolates Differing in Organoleptic Properties: A GC-O Comparative Study. Molecules 2020, 25, 1809. [Google Scholar] [CrossRef] [Green Version]
- Diez-Simon, C.; Mumm, R.; Hall, R.D. Mass Spectrometry-Based Metabolomics of Volatiles as a New Tool for Understanding Aroma and Flavour Chemistry in Processed Food Products. Metabolomics 2019, 15, 41. [Google Scholar] [CrossRef] [Green Version]
- Frauendorfer, F.; Schieberle, P. Identification of the Key Aroma Compounds in Cocoa Powder Based on Molecular Sensory Correlations. J. Agric. Food Chem. 2006, 54, 5521–5529. [Google Scholar] [CrossRef]
- Mansurova, M.; Ebert, B.; Blank, L.; Ibáñez, A. A Breath of Information: The Volatilome. Curr. Genet. 2017, 64, 959–964. [Google Scholar] [CrossRef]
- Calla-Quispe, E.; Fuentes-Rivera, H.L.; Ramírez, P.; Martel, C.; Ibañez, A.J. Mass Spectrometry: A Rosetta Stone to Learn How Fungi Interact and Talk. Life 2020, 10, 89. [Google Scholar] [CrossRef]
- Esparza, E.; Hadzich, A.; Kofer, W.; Mithöfer, A.; Cosio, E.G. Bioactive maca (Lepidium meyenii) alkamides are a result of traditional Andean postharvest drying practices. Phytochemistry. 2015, 116, 138–148. [Google Scholar] [CrossRef] [Green Version]
- Caligiani, A.; Cirlini, M.; Palla, G.; Ravaglia, R.; Arlorio, M. GC-MS Detection of Chiral Markers in Cocoa Beans of Different Quality and Geographic Origin. Chirality 2007, 19, 329–334. [Google Scholar] [CrossRef]
- del Rosario Brunetto, M.; Cayama, Y.D.; Gutiérrez, L.; Roa, S.C.; Méndez, Y.C.; Gallignani, M.; Zambrano, A.; Gómez, Á.; Ramos, G. Headspace Gas Chromatography–Mass Spectrometry Determination of Alkylpyrazines in Cocoa Liquor Samples. Food Chem. 2009, 112, 253–257. [Google Scholar] [CrossRef]
- Magi, E.; Bono, L.; di Carro, M. Characterization of Cocoa Liquors by GC-MS and LC-MS/MS: Focus on Alkylpyrazines and Flavanols: Alkylpyrazines and Flavanols in Cocoa Liquors. J. Mass Spectrom. 2012, 47, 1191–1197. [Google Scholar] [CrossRef]
- Michel, S.; Ibañez, A.J.; Mansurova, M. Investigation of Compounds Responsible for the Flavor of Peruvian Chocolate from Fine Flavor Cocoa. In Proceedings of the 15th Annual Conference of the Metabolomics Society, The Hague, The Netherlands, 23–27 June 2019. [Google Scholar]
- Humston, E.M.; Knowles, J.D.; McShea, A.; Synovec, R.E. Quantitative Assessment of Moisture Damage for Cacao Bean Quality Using Two-Dimensional Gas Chromatography Combined with Time-of-Flight Mass Spectrometry and Chemometrics. J. Chromatogr. A 2010, 1217, 1963–1970. [Google Scholar] [CrossRef]
- Meersman, E.; Steensels, J.; Struyf, N.; Paulus, T.; Saels, V.; Mathawan, M.; Allegaert, L.; Vrancken, G.; Verstrepen, K.J. Tuning Chocolate Flavor through Development of Thermotolerant Saccharomyces Cerevisiae Starter Cultures with Increased Acetate Ester Production. Appl. Environ. Microbiol. 2016, 82, 732–746. [Google Scholar] [CrossRef] [Green Version]
- Bonvehí, J.S. Investigation of Aromatic Compounds in Roasted Cocoa Powder. Eur. Food Res. Technol. 2005, 221, 19–29. [Google Scholar] [CrossRef]
- Stewart, A.; Grandison, A.S.; Ryan, A.; Festring, D.; Methven, L.; Parker, J.K. Impact of the Skim Milk Powder Manufacturing Process on the Flavor of Model White Chocolate. J. Agric. Food Chem. 2017, 65, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Hinneh, M.; van de Walle, D.; Tzompa-Sosa, D.A.; Haeck, J.; Abotsi, E.E.; de Winne, A.; Messens, K.; van Durme, J.; Afoakwa, E.O.; de Cooman, L.; et al. Comparing Flavor Profiles of Dark Chocolates Refined with Melanger and Conched with Stephan Mixer in Various Alternative Chocolate Production Techniques. Eur. Food Res. Technol. 2019, 245, 837–852. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 5th ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Rodriguez-Campos, J.; Escalona-Buendía, H.B.; Orozco-Avila, I.; Lugo-Cervantes, E.; Jaramillo-Flores, M.E. Dynamics of Volatile and Non-Volatile Compounds in Cocoa (Theobroma Cacao, L.) during Fermentation and Drying Processes Using Principal Components Analysis. Food Res. Int. 2011, 44, 250–258. [Google Scholar] [CrossRef]
- Tuenter, E.; Delbaere, C.; de Winne, A.; Bijttebier, S.; Custers, D.; Foubert, K.; van Durme, J.; Messens, K.; Dewettinck, K.; Pieters, L. Non-Volatile and Volatile Composition of West African Bulk and Ecuadorian Fine-Flavor Cocoa Liquor and Chocolate. Food Res. Int. 2020, 130, 108943. [Google Scholar] [CrossRef]
- Providing Information for the Flavor, Fragrance, Food and Cosmetic Industries. Available online: http://www.thegoodscentscompany.com/ (accessed on 24 November 2020).
- Braga, S.C.G.N.; Oliveira, L.F.; Hashimoto, J.C.; Gama, M.R.; Efraim, P.; Poppi, R.J.; Augusto, F. Study of Volatile Profile in Cocoa Nibs, Cocoa Liquor and Chocolate on Production Process Using GC × GC-QMS. Microchem. J. 2018, 141, 353–361. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Ryan, A. Matrix Effects on Flavour Volatiles Release in Dark Chocolates Varying in Particle Size Distribution and Fat Content Using GC–Mass Spectrometry and GC–Olfactometry. Food Chem. 2009, 113, 208–215. [Google Scholar] [CrossRef]
- Moshonas, M.G.; Shaw, P.E.; Baldwin, E.A.; Yuen, W. Volatile and Nonvolatile Components in Hami Melon (Cucumis Melo, L.). LWT Food Sci. Technol. 2013, 26, 577–589. [Google Scholar] [CrossRef]
- Owusu, M.; Petersen, M.A.; Heimdal, H. Effect of Fermentation Method, Roasting and Conching Conditions on the Aroma Volatiles of Dark Chocolate: Processing Conditions on Heap and Tray Chocolates. J. Food Process. Preserv. 2012, 36, 446–456. [Google Scholar] [CrossRef]
- Ramos, C.L.; Dias, D.R.; Miguel, M.G.D.C.P.; Schwan, R.F. Impact of Different Cocoa Hybrids (Theobroma Cacao, L.) and S. Cerevisiae UFLA CA11 Inoculation on Microbial Communities and Volatile Compounds of Cocoa Fermentation. Food Res. Int. 2014, 64, 908–918. [Google Scholar] [CrossRef]
- Cutzach, I.; Chatonnet, P.; Henry, R.; Dubourdieu, D. Identification of Volatile Compounds with a “Toasty” Aroma in Heated Oak Used in Barrelmaking. J. Agric. Food Chem. 1997, 45, 2217–2224. [Google Scholar] [CrossRef]
- Su, G.; Zheng, L.; Cui, C.; Yang, B.; Ren, J.; Zhao, M. Characterization of Antioxidant Activity and Volatile Compounds of Maillard Reaction Products Derived from Different Peptide Fractions of Peanut Hydrolysate. Food Res. Int. 2011, 44, 3250–3258. [Google Scholar] [CrossRef]
- Augustyn, O.P.H.; Wyk, C.J.V.; Muller, C.J.; Kepner, R.E.; Webb, A.D. The Structure of Solerone [5-Acetyldihydro-2(3 H )-Furanone], a Substituted 7-Lactone Involved in Wine Aroma. J. Agric. Food Chem. 1971, 19, 1128–1130. [Google Scholar] [CrossRef]
- Bullard, R.W.; Leiker, T.J.; Peterson, J.E.; Kilburn, S.R. Volatile Components of Fermented Egg, an Animal Attractant and Repellent. J. Agric. Food Chem. 1978, 26, 155–159. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Panigrahi, S. Solid-Phase Microextraction (SPME) Techniques for Quality Characterization of Food Products: A Review. Food Bioprocess Technol. 2011, 4, 1–26. [Google Scholar] [CrossRef]
- Ducki, S.; Miralles-Garcia, J.; Zumbé, A.; Tornero, A.; Storey, D.M. Evaluation of Solid-Phase Micro-Extraction Coupled to Gas Chromatography–Mass Spectrometry for the Headspace Analysis of Volatile Compounds in Cocoa Products. Talanta 2008, 74, 1166–1174. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Flamini, G.; Tessieri, C.; Pistelli, L. From the Raw Seed to Chocolate: Volatile Profile of Blanco de Criollo in Different Phases of the Processing Chain. Microchem. J. 2017, 133, 474–479. [Google Scholar] [CrossRef]
- Magalhães da Veiga Moreira, I.; de Figueiredo Vilela, L.; da Cruz Pedroso Miguel, M.; Santos, C.; Lima, N.; Freitas Schwan, R. Impact of a Microbial Cocktail Used as a Starter Culture on Cocoa Fermentation and Chocolate Flavor. Molecules 2017, 22, 766. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Woo, J.; Pae, A.N.; Um, M.Y.; Cho, N.-C.; Park, K.D.; Yoon, M.; Kim, J.; Lee, C.J.; Cho, S. α-Pinene, a Major Constituent of Pine Tree Oils, Enhances Non-Rapid Eye Movement Sleep in Mice through GABAA-Benzodiazepine Receptors. Mol. Pharmacol. 2016, 90, 530–539. [Google Scholar] [CrossRef] [Green Version]
- Chitarrini, G.; Dordevic, N.; Guerra, W.; Robatscher, P.; Lozano, L. Aroma Investigation of New and Standard Apple Varieties Grown at Two Altitudes Using Gas Chromatography-Mass Spectrometry Combined with Sensory Analysis. Molecules 2020, 25, 3007. [Google Scholar] [CrossRef]
- Ahn, Y.-Y.; Ahnert, S.E.; Bagrow, J.P.; Barabási, A.-L. Flavor Network and the Principles of Food Pairing. Sci. Rep. 2011, 1, 196. [Google Scholar] [CrossRef] [PubMed]
- Januszewska, R. Hidden Persuaders in Cocoa and Chocolate, a Flavor Lexicon for Cocoa and Chocolate Sensory Professionals, 1st ed.; Woodhead Publishing: Cambridge, UK, 2018. [Google Scholar]
- Kadow, D.; Bohlmann, J.; Phillips, W.; Lieberei, R. Identification of Main Fine or Flavour Components in Two Genotypes of the Cocoa Tree (Theobroma Cacao L.). J. Appl. Bot. Food Qual. 2013, 86. [Google Scholar] [CrossRef]
- Barišić, V.; Kopjar, M.; Jozinović, A.; Flanjak, I.; Ačkar, Đ.; Miličević, B.; Šubarić, D.; Jokić, S.; Babić, J. The Chemistry behind Chocolate Production. Molecules 2019, 24, 3163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stark, T.; Bareuther, S.; Hofmann, T. Molecular Definition of the Taste of Roasted Cocoa Nibs (Theobroma Cacao) by Means of Quantitative Studies and Sensory Experiments. J. Agric. Food Chem. 2006, 54, 5530–5539. [Google Scholar] [CrossRef] [PubMed]
- Rocha, I.S. Effect of the Roasting Temperature and Time of Cocoa Beans on the Sensory Characteristics and Acceptability of Chocolate. Food Sci. Technol. Campinas 2017, 37, 522–530. [Google Scholar] [CrossRef] [Green Version]
- Spada, F.P.; Zerbeto, L.M.; Ragazi, G.B.C.; Maria, É.; Gutierrez, R.; Souza, M.C.D.; Parker, J.K.; Brazaca, S.G.C. Optimization of Postharvest Conditions to Produce Chocolate Aroma from Roasted Jackfruit Seeds. J. Agric. Food Chem. 2017, 65, 1196–1208. [Google Scholar] [CrossRef]
- Owusu, M.; Petersen, M.A.; Heimdal, H. Relationship of Sensory and Instrumental Aroma Measurements of Dark Chocolate as Influenced by Fermentation Method, Roasting and Conching Conditions. J. Food Sci. Technol. 2013, 50, 909–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Retention Time (min) | Volatile Organic Compound | Odor Description | Fragments (m/z) | Ref. a |
|---|---|---|---|---|
| Acids | ||||
| 8.046 | Acetic acid | Sour, astringent, vinegar | 60, 61 | [16,27] |
| 11.772 | Propanoic acid | Pungent, rancid, soy | 74, 73 | [27,36] |
| 12.807 | Isobutyric acid | Rancid, butter, cheese | 73, 88 | [16,37] |
| 14.527 | Butanoic acid | Rancid, cheese, sweat | 60, 73 | [27,36,38] |
| 15.651 | Isovaleric acid | Sweat, acid, rancid | 60, 87 | [16,37,39] |
| 22.080 | 3-Hydroxybutanoic acid | 59, 71 | ||
| Alcohols and Phenols | ||||
| 5.049 | 1-Butanol | Fusel, dry | 56, 55 | [40] |
| 6.191 | 1-Ethoxy-2-propanol | 59, 61 | ||
| 9.608 | 3-Methyl-2nitrobenzyl alcohol | 104, 93 | ||
| 11.280 | 2-Heptanol | Citrusy, floral | 55, 83 | [16,41,42] |
| 12.902 | 2,4-Dimethyl-3-pentanol | 73, 55 | ||
| 14.206 | 3-Octenol | Earthy, green, oily b | 57, 55 | [43] |
| 14.409 | 4-Cyclohexene-1,2-diol | 60, 96 | ||
| 16.991 | 1-Octanol | Fatty, waxy | 55, 56 | [37,44] |
| 18.049 | cis-3,3,5-Trimethylcyclohexanol | Minty b | 83, 109 | [43] |
| 18.155 | 1-Methoxy-2-butanol | 59, 58 | ||
| 18.317 | 2,4-Dimethyl-3-pentanol | 73, 55 | ||
| 18.730 | 6-Methyl-1-octanol | 55, 105 | ||
| 19.935 | 3-Methoxy-1-butanol | 59, 71 | ||
| 22,386 | Guaiacol | Smoked, sweet | 109, 124 | [16,45] |
| 22.570 | Benzyl alcohol | Sweet, flower | 79, 77 | [14,39,41] |
| 23.369 | α-Phenethyl alcohol | Sweet, floral | 79, 107 | [14,16] |
| 27.075 | 2,2-Dimethyl-1,3-propanediol | 56, 55 | ||
| Aldehydes and Ketones | ||||
| 2.671 | 3-Methyl-2-heptanone | 72, 57 | ||
| 2.935 | Isovaleraldehyde | Malty | 58, 57 | [45] |
| 3.767 | Pentanal | Warm, fruity, nutty | 58, 57 | [14,40,44] |
| 6.548 | Hexanal | Green | 56, 57 | [14,44,45] |
| 9.793 | Heptanal | Fatty, harsh, pungent | 57, 70 | [14,40] |
| 10.027 | 2-Heptanone | Fruity, spicy, cinnamon | 58, 71 | [9,14,40] |
| 15.739 | Nonanal | Soapy | 57, 56 | [14,44,45] |
| 15.874 | 2-Nonanone | Milk, green, fruity | 58, 57 | [14,36,39] |
| 17.867 | 2-Cyclopentene-1,4-dione | 96, 68 | ||
| 20.097 | Acetophenone | Flower, almond, sweet | 105, 77 | [9,37,39] |
| 20.790 | 2-Undecanone | Fruity, waxy | 58, 60 | [15] |
| 21.434 | 3-Methylcyclopentane-1,2-dione | Caramel, maple b | 112, 55 | [43] |
| 23.267 | 3,4-Dihydroxy-3,4-dimethyl-2,5-hexanedione | 88,89 | ||
| Esters | ||||
| 5.469 | Butyl acetate | Fruity, pineapple | 56, 57 | [9,40] |
| 7.228 | Isoamyl acetate | Fruity, sweet, banana | 70, 55 | [39,40] |
| 15.126 | (2E)-2-Butenyl propionate | 57, 75 | ||
| 17.346 | Isobutyl lactate | Buttery, caramelly b | 57, 75 | [43] |
| 18.239 | 2,3-Butanedioldiacetate | Fruity | 87, 72 | [46] |
| 19.309 | 2-Butoxyethyl acetate | Sweet, fruity b | 55, 88 | [43] |
| 20.176 | Ethyl benzoate | Sweet, fruity, cherry | 105, 77 | [15] |
| 20.591 | an isomer of Ethyl isobutyrate | Ethereal sweet, fruity | 71, 116 | [36] |
| 20.824 | an isomer of Ethyl isobutyrate | Ethereal sweet, fruity | 71, 116 | [36] |
| 21.994 | 2-Ethoxyethyl-3 -methylbutanoate | 85, 57 | ||
| 22.156 | Methyl benzeneacetate | Sweet, floral, honey b | 91, 150 | [43] |
| 24.247 | 1-Methylbutyl benzoate | Sweet, fruity b | 105, 123 | [43] |
| 24.578 | Phenethyl acetate | Sweet, floral | 91, 104 | [45] |
| 25.206 | Propanoic acid, 2-methyl-2,2-dimethyl-1-(2-hydroxy-1-methylethyl) propyl ester | 71, 83 | ||
| 25.616 | Propanoic acid, 2-methyl-,3-hydroxy-2,4,4-trimethylpentyl ester | 71,56 | ||
| 29.117 | Triacetin | Creamy, slightly acidic b | 103, 145 | [43] |
| 30.001 | Ethyl-4-ethoxybenzoate | 121, 149 | ||
| 30.689 | Piperidin-2-one-5-carboxylic acid,5,6-didehydro-,methyl ester | 155, 124 | ||
| Furans, furanones, pyrans, pyrones | ||||
| 9.281 | 2-Pentylfuran | Musty, green | 81, 82 | [47] |
| 17.222 | Furfuryl alcohol | Faint burning | 98, 97 | [48] |
| 27.307 | 3,5-Dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one | Roasty, caramel | 144, 101 | [49] |
| 31.019 | 2,3-Dihydrobenzofuran | Sweet, herbal, floral | 120, 91 | [50] |
| 31.294 | 5-Acetyldihydro-2(3H)-furanone | Wine | 85, 57 | [51] |
| Hydrocarbons | ||||
| 3.515 | 2,2,4,6,6-Pentamethylheptane | 57, 56 | ||
| 3.939 | Toluene | Sweet | 91, 92 | [45] |
| 6.725 | Undecane | 57, 71 | ||
| 11.866 | 1-Methyl-3-propylbenzene | Off odor | 105, 74 | [52] |
| Lactones | ||||
| 21.750 | Butyrolactone | Faint, sweet, buttery | 86, 56 | [40,42] |
| 22.097 | δ-Valerolactone | Herbal, woody b | 56, 100 | [43] |
| 27.792 | Pantolactone | Cotton candy b | 71, 57 | [43] |
| 29.721 | δ-Octalactone | Sweet, coconut, creamy b | 99, 71 | [43] |
| 30.254 | Dehydromevalonic lactone | 88, 112 | ||
| Nitrogen Compounds | ||||
| 25.746 | Benzyl nitrile | Bitter, almonds, spicy b | 117, 60 | [43] |
| Pyrazines, piperazines | ||||
| 9.422 | Methylpyrazine | Nutty, chocolate, cocoa | 94, 67 | [36,37,47] |
| 11.557 | 2,5-Dimethylpyrazine | Cocoa, sweet chocolate, roasted nuts | 108, 81 | [45,51,52,53,54] |
| 12.200 | 2,3-Dimethylpyrazine | Caramel, sweet chocolate, cocoa | 108, 67 | [9,37,40,51,52,53,54] |
| 13.413 | 2-Ethyl-6-methyl pyrazine | Cocoa, roasted, green | 121, 122 | [45] |
| 13.685 | 2-Ethyl-5-methylpyrazine | Nutty, raw potato, herbal, roasted | 121, 122 | [37,47] |
| 13.838 | Trimethylpyrazine | Cocoa, roasted nuts, sweet | 122, 81 | [9,36,39,45] |
| 15.005 | 3-Ethyl-2,5-dimethylpyrazine | Earthy | 135, 136 | [47] |
| 16.442 | 3,5-Diethyl-2-methyl pyrazine | Cocoa, chocolate, sweet | 149, 150 | [45] |
| 16.611 | Tetramethylpyrazine | Roasted, green, coffee, cocoa | 54, 136 | [36,39,45,55,56] |
| 18.915 | 8-Methyl-1,2,4-triazolo [4,3-b] pyridazine | 52, 134 | ||
| 20.341 | 2-(3-Methylbutyl)-3,5-dimethylpyrazine | 122, 135 | ||
| 21.209 | (E)-5-Methyl-2-(1-propenyl) pyrazine | 134, 133 | ||
| 22.846 | 2-Acetyl-3,5-dimethylpyrazine | Roasted, hazelnuts | 60, 53 | [40] |
| Pyridines | ||||
| 22.774 | N-Acetyl-4H-pyridine | 80, 123 | ||
| 24.735 | 1-Acetyl-1,2,3,4-tetrahydropyridine | 82, 83 | ||
| Pyrrol | ||||
| 6.500 | 1-Methylpyrrole | Woody b | 81, 80 | [43] |
| 24.060 | 2-Acetylpyrrole | Cocoa, hazelnut, Bread, licorice | 94, 109 | [36,45] |
| 27.241 | 1-Methylpyrrole-2-carboxaldehyde | Roasted nutty b | 109, 108 | [43] |
| 29.640 | 1-Methyl-1,5-dihydro-2H-pyrrol-2-one | Ammoniacal b | 97, 60 | [43] |
| Sulfur Compounds | ||||
| 12.424 | Dimethyl trisulfide | Sulfurous | 126, 79 | [45] |
| 26.072 | Dimethyl sulfone | Sulfurous b | 79, 94 | [43] |
| Terpenes, terpenoids | ||||
| 7.579 | α-Pinene | Woody, herbal | 93, 69 | [57] |
| 17.071 | Linalool | Flowery, floral, fruity, lavender | 71, 93 | [9,15,16,37,40,42,52] |
| 22.516 | Epoxylinalool | Sweet, honey | 68, 94 | [45] |
| Retention Time (min) | Fragments (m/z) | Molecular Formula | Volatile Organic Compound | Odor Description |
|---|---|---|---|---|
| 16.611 | 54, 136 | C8H12N2 | Tetramethylpyrazine a | Roasted, green, coffee, cocoa |
| 17.346 | 57, 75 | C7H14O3 | Isobutyl lactate | Buttery, caramelly |
| 18.915 | 52, 134 | C6H6N4 | 8-Methyl-1,2,4-triazolo[4,3-b] pyridazine | No flavor information |
| 19.309 | 55, 88 | C8H16O3 | 2-Butoxyethyl acetate | Sweet, fruity |
| 20.591 | 71, 116 | C6H12O2 | an isomer of Ethyl isobutyrate | Ethereal sweet, fruity |
| 20.824 | 71, 116 | C6H12O2 | an isomer of Ethyl isobutyrate | Ethereal sweet, fruity |
| 22.080 | 59, 71 | C4H8O3 | 3-Hydroxybutanoic acid | No flavor information |
| 22.516 | 68, 94 | C10H18O2 | Epoxylinalol | Sweet, honey |
| 23.267 | 88, 89 | C8H14O4 | 3,4-Dihydroxy-3,4-dimethyl-2,5-hexanedione a | No flavor information |
| 23.369 | 79, 107 | C8H10O | α-Phenethyl alcohol | Sweet, floral |
| 24.578 | 91, 104 | C10H12O2 | Phenethyl acetate a | Sweet, floral |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michel, S.; Baraka, L.F.; Ibañez, A.J.; Mansurova, M. Mass Spectrometry-Based Flavor Monitoring of Peruvian Chocolate Fabrication Process. Metabolites 2021, 11, 71. https://doi.org/10.3390/metabo11020071
Michel S, Baraka LF, Ibañez AJ, Mansurova M. Mass Spectrometry-Based Flavor Monitoring of Peruvian Chocolate Fabrication Process. Metabolites. 2021; 11(2):71. https://doi.org/10.3390/metabo11020071
Chicago/Turabian StyleMichel, Stephanie, Luka Franco Baraka, Alfredo J. Ibañez, and Madina Mansurova. 2021. "Mass Spectrometry-Based Flavor Monitoring of Peruvian Chocolate Fabrication Process" Metabolites 11, no. 2: 71. https://doi.org/10.3390/metabo11020071
APA StyleMichel, S., Baraka, L. F., Ibañez, A. J., & Mansurova, M. (2021). Mass Spectrometry-Based Flavor Monitoring of Peruvian Chocolate Fabrication Process. Metabolites, 11(2), 71. https://doi.org/10.3390/metabo11020071