Goldfish Response to Chronic Hypoxia: Mitochondrial Respiration, Fuel Preference and Energy Metabolism
Abstract
:1. Introduction
2. Results
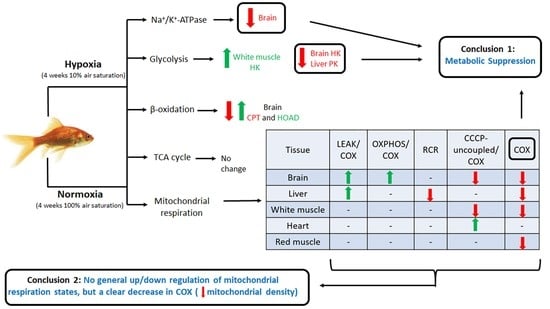
2.1. Mitochondrial Respiration
2.1.1. LEAK
2.1.2. OXPHOS
2.1.3. CCCP-Uncoupled State
2.1.4. Cytochrome Oxidase
2.2. Energy Metabolism Enzymes
2.3. Na+/K+-ATPase
3. Discussion
3.1. Effects of Hypoxia on Mitochondrial Respiration
3.2. Tissue-Secific Fuel Preference of Goldfish Mitochondria
3.3. Chronic Hypoxia and Glycolysis
3.4. β-Oxidation and TCA Cycle
3.5. Downregulation of Na+/K+-ATPase in Goldfish Brain
4. Methods
4.1. Animals
4.2. Mitochondrial Respiration
4.3. Enzyme Assays
4.4. Calculations and Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bickler, P.E.; Buck, L.T. Hypoxia tolerance in reptiles, amphibians, and fishes: Life with variable oxygen availability. Annu. Rev. Physiol. 2007, 69, 145–170. [Google Scholar] [CrossRef]
- Semenza, G.L. Life with oxygen. Science 2007, 318, 62–64. [Google Scholar] [CrossRef]
- Solaini, G.; Baracca, A.; Lenaz, G.; Sgarbi, G. Hypoxia and mitochondrial oxidative metabolism. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2010, 1797, 1171–1177. [Google Scholar] [CrossRef] [Green Version]
- Boutilier, R.G. Mechanisms of cell survival in hypoxia and hypothermia. J. Exp. Biol. 2001, 204, 3171–3181. [Google Scholar] [PubMed]
- Martínez, M.L.; Landry, C.; Boehm, R.; Manning, S.; Cheek, A.O.; Rees, B.B. Effects of long-term hypoxia on enzymes of carbohydrate metabolism in the gulf killifish, fundulus grandis. J. Exp. Biol. 2006, 209, 3851–3861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochachka, P.W. Defense strategies against hypoxia and hypothermia. Science 1986, 231, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Pamenter, M.E. Mitochondria: A multimodal hub of hypoxia tolerance. Can. J. Zool. 2014, 92, 569–589. [Google Scholar] [CrossRef]
- Hickey, A.J.R.; Renshaw, G.M.C.; Speers-Roesch, B.; Richards, J.G.; Wang, Y.; Farrell, A.P.; Brauner, C.J. A radical approach to beating hypoxia: Depressed free radical release from heart fibres of the hypoxia-tolerant epaulette shark (hemiscyllum ocellatum). J. Comp. Physiol. B 2012, 182, 91–100. [Google Scholar] [CrossRef]
- Sokolova, I. Mitochondrial adaptations to variable environments and their role in animals’ stress tolerance. Integr. Comp. Biol. 2018, 58, 519–531. [Google Scholar] [CrossRef]
- St-Pierre, J.; Tattersall, G.J.; Boutilier, R.G. Metabolic depression and enhanced o2 affinity of mitochondria in hypoxic hypometabolism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R1205–R1214. [Google Scholar] [CrossRef]
- Du, S.N.; Mahalingam, S.; Borowiec, B.G.; Scott, G.R. Mitochondrial physiology and reactive oxygen species production are altered by hypoxia acclimation in killifish (fundulus heteroclitus). J. Exp. Biol. 2016, 219, 1130–1138. [Google Scholar] [CrossRef] [Green Version]
- Cook, D.G.; Iftikar, F.I.; Baker, D.W.; Hickey, A.J.; Herbert, N.A. Low-o2 acclimation shifts the hypoxia avoidance behaviour of snapper (pagrus auratus) with only subtle changes in aerobic and anaerobic function. J. Exp. Biol. 2013, 216, 369–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farhat, E.; Turenne, E.D.; Choi, K.; Weber, J.-M. Hypoxia-induced remodelling of goldfish membranes. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2019, 237. [Google Scholar] [CrossRef] [PubMed]
- Van Waversveld, J.; Addink, A.; van den Thillart, G. The anaerobic energy metabolism of goldfish determined by simultaneous direct and indirect calorimetry during anoxia and hypoxia. J. Comp. Physiol. B 1989, 159, 263–268. [Google Scholar] [CrossRef]
- Van Ginneken, V.J.; Snelderwaard, P.; van der Linden, R.; van der Reijden, N.; van den Thillart, G.E.; Kramer, K. Coupling of heart rate with metabolic depression in fish: A radiotelemetric and calorimetric study. Thermochim. Acta 2004, 414, 1–10. [Google Scholar] [CrossRef]
- Richards, J.G. Metabolic and molecular responses of fish to hypoxia. In Fish Physiology; Elsevier: Cambridge, MA, USA, 2009; Volume 27, pp. 443–485. [Google Scholar]
- Jibb, L.A.; Richards, J.G. Amp-activated protein kinase activity during metabolic rate depression in the hypoxic goldfish, carassius auratus. J. Exp. Biol. 2008, 211, 3111–3122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, J.-M. Revealing how goldfish defy anoxia. J. Exp. Biol. 2016, 219, 1422–1423. [Google Scholar] [CrossRef] [Green Version]
- Van den Thillart, G.; Berge-Henegouwen, M.; Kesbeke, F. Anaerobic metabolism of goldfish, Carassius auratus (l.): Ethanol and CO2 excretion rates and anoxia tolerance at 20, 10, and 5 °C. Comp. Biochem. Physiol. 1983, 76A, 295–300. [Google Scholar] [CrossRef]
- McElroy, G.S.; Chandel, N.S. Mitochondria control acute and chronic responses to hypoxia. Exp. Cell Res. 2017, 356, 217–222. [Google Scholar] [CrossRef]
- St-Pierre, J.; Brand, M.D.; Boutilier, R.G. Mitochondria as atp consumers: Cellular treason in anoxia. Proc. Natl. Acad. Sci. USA 2000, 97, 8670–8674. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Rohr, K. Oxygen is the high-energy molecule powering complex multicellular life: Fundamental corrections to traditional bioenergetics. ACS Omega 2020, 5, 2221–2233. [Google Scholar] [CrossRef]
- Pamenter, M.E.; Gomez, C.R.; Richards, J.G.; Milsom, W.K. Mitochondrial responses to prolonged anoxia in brain of red-eared slider turtles. Biol. Lett. 2016, 12. [Google Scholar] [CrossRef]
- Gomez, C.R.; Richards, J.G. Mitochondrial responses to anoxia exposure in red eared sliders (trachemys scripta). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2018, 224, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Quinlan, C.L.; Perevoschikova, I.V.; Goncalves, R.L.; Hey-Mogensen, M.; Brand, M.D. The determination and analysis of site-specific rates of mitochondrial reactive oxygen species production. In Methods in Enzymology; Elsevier: Waltham, MA, USA, 2013; Volume 526, pp. 189–217. [Google Scholar]
- Weber, J.M.; Haman, F. Oxidative fuel selection: Adjusting mix and flux to stay alive. In Animals and Environments; Morris, S., Vosloo, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 1275, pp. 22–31. [Google Scholar]
- Bundgaard, A.; Qvortrup, K.; Rasmussen, L.J.; Fago, A. Turtles maintain mitochondrial integrity but reduce mitochondrial respiratory capacity in the heart after cold acclimation and anoxia. J. Exp. Biol. 2019, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Thillart, G.; Kesbeke, F.; van Waarde, A. Anaerobic energy-metabolism of goldfish, Carassius auratus (l.). J. Comp. Physiol. 1980, 136, 45–52. [Google Scholar] [CrossRef]
- Regan, M.D.; Gill, I.S.; Richards, J.G. Calorespirometry reveals that goldfish prioritize aerobic metabolism over metabolic rate depression in all but near-anoxic environments. J. Exp. Biol. 2017, 220, 564–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, I.A.; Bernard, L.M. Ultrastructure and metabolism of skeletal muscle fibres in the tench: Effects of long-term acclimation to hypoxia. Cell Tissue Res. 1982, 227, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Van den Thillart, G.; Smit, H. Carbohydrate metabolism of goldfish (Carassius auratus l.)–Effects of long-term hypoxia-acclimation on enzyme patterns of red muscle, white muscle and liver. J. Comp. Physiol. B 1984, 154, 477–486. [Google Scholar] [CrossRef]
- Mahfouz, M.E.; Hegazi, M.M.; El-Magd, M.A.; Kasem, E.A. Metabolic and molecular responses in nile tilapia, oreochromis niloticus during short and prolonged hypoxia. Mar. Freshw. Behav. Physiol. 2015, 48, 319–340. [Google Scholar] [CrossRef]
- Zhou, B.; Wu, R.; Randall, D.; Lam, P.; Ip, Y.; Chew, S. Metabolic adjustments in the common carp during prolonged hypoxia. J. Fish Biol. 2000, 57, 1160–1171. [Google Scholar] [CrossRef]
- Pillet, M.; Dupont-Prinet, A.; Chabot, D.; Tremblay, R.; Audet, C. Effects of exposure to hypoxia on metabolic pathways in northern shrimp (pandalus borealis) and greenland halibut (reinhardtius hippoglossoides). J. Exp. Mar. Biol. Ecol. 2016, 483, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Xu, X.; Li, W.; Zhang, X. Effects of acute and chronic hypoxia on the locomotion and enzyme of energy metabolism in chinese shrimp fenneropenaeus chinensis. Mar. Freshw. Behav. Physiol. 2018, 51, 275–291. [Google Scholar] [CrossRef]
- Gerber, L.; Clow, K.A.; Katan, T.; Emam, M.; Leeuwis, R.H.; Parrish, C.C.; Gamperl, A.K. Cardiac mitochondrial function, nitric oxide sensitivity and lipid composition following hypoxia acclimation in sablefish. J. Exp. Biol. 2019, 222. [Google Scholar] [CrossRef]
- Farhat, E.; Devereaux, M.E.M.; Pamenter, M.E.; Weber, J.-M. Naked mole-rats suppress energy metabolism and modulate membrane cholesterol in chronic hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 319, R148–R155. [Google Scholar] [CrossRef] [PubMed]
- Hylland, P.; Milton, S.; Pek, M.; Nilsson, G.E.; Lutz, P.L. Brain na+/k+-atpase activity in two anoxia tolerant vertebrates: Crucian carp and freshwater turtle. Neurosci. Lett. 1997, 235, 89–92. [Google Scholar] [CrossRef]
- Soengas, J.L.; Aldegunde, M. Energy metabolism of fish brain. Comp. Biochem. Physiol. B 2002, 131, 271–296. [Google Scholar] [CrossRef]
- Erecińska, M.; Silver, I.A. Ions and energy in mammalian brain. Prog. Neurobiol. 1994, 43, 37–71. [Google Scholar] [CrossRef]
- Bickler, P.E.; Buck, L.T. Adaptations of vertebrate neurons to hypoxia and anoxia: Maintaining critical ca2+ concentrations. J. Exp. Biol. 1998, 201, 1141–1152. [Google Scholar]
- Boutilier, R.; St-Pierre, J. Surviving hypoxia without really dying. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2000, 126, 481–490. [Google Scholar] [CrossRef]
- Wilkie, M.P.; Pamenter, M.E.; Alkabie, S.; Carapic, D.; Shin, D.S.H.; Buck, L.T. Evidence of anoxia-induced channel arrest in the brain of the goldfish (carassius auratus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008, 148, 355–362. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Strobl, D.; Ruttmann, E.; Königsrainer, A.; Margreiter, R.; Gnaiger, E. Evaluation of mitochondrial respiratory function in small biopsies of liver. Anal. Biochem. 2002, 305, 186–194. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Veksler, V.; Gellerich, F.N.; Saks, V.; Margreiter, R.; Kunz, W.S. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat. Protoc. 2008, 3. [Google Scholar] [CrossRef]
- Salin, K.; Auer, S.K.; Rudolf, A.M.; Anderson, G.J.; Selman, C.; Metcalfe, N.B. Variation in metabolic rate among individuals is related to tissue-specific differences in mitochondrial leak respiration. Physiol. Biochem. Zool. 2016, 89, 511–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, S.; Kraunsøe, R.; Gram, M.; Gnaiger, E.; Helge, J.W.; Dela, F. The best approach: Homogenization or manual permeabilization of human skeletal muscle fibers for respirometry? Anal. Biochem. 2014, 446, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Velasco, C.; Draxl, A.; Wiethuchter, A.; Eigentler, A.; Gnaiger, E. Mitochondrial respiration in permeabilized fibres versus homogenate from trout heart and liver. Mitochondrial Physiol. Netw. 2012, 17, 1–12. [Google Scholar]
- Pesta, D.; Gnaiger, E. High-resolution respirometry: Oxphos protocols for human cells and permeabilized fibers from small biopsies of human muscle. In Mitochondrial Bioenergetics; Springer: Totowa, NJ, USA, 2012; pp. 25–58. [Google Scholar]
- Bourguignon, A.; Rameau, A.; Toullec, G.; Romestaing, C.; Roussel, D. Increased mitochondrial energy efficiency in skeletal muscle after long-term fasting: Its relevance to animal performance. J. Exp. Biol. 2017, 220, 2445–2451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, S.; Nielsen, J.; Hansen, C.N.; Nielsen, L.B.; Wibrand, F.; Stride, N.; Schroder, H.D.; Boushel, R.; Helge, J.W.; Dela, F.; et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J. Physiol. 2012, 590, 3349–3360. [Google Scholar] [CrossRef] [PubMed]
- Best, C.; Melnyk-Lamont, N.; Gesto, M.; Vijayan, M.M. Environmental levels of the antidepressant venlafaxine impact the metabolic capacity of rainbow trout. Aquat. Toxicol. 2014, 155, 190–198. [Google Scholar] [CrossRef]
- Zammit, V.A.; Beis, I.; Newsholme, E.A. Maximum activities and effects of fructose bisphosphate on pyruvate kinase from muscles of vertebrates and invertebrates in relation to the control of glycolysis. Biochem. J. 1978, 174, 989–998. [Google Scholar] [CrossRef] [Green Version]
- Zammit, V.A.; Newsholme, E.A. The maximum activities of hexokinase, phosphorylase, phosphofructokinase, glycerol phosphate dehydrogenases, lactate dehydrogenase, octopine dehydrogenase, phosphoenolpyruvate carboxykinase, nucleoside diphosphatekinase, glutamate-oxaloacetate transaminase and arginine kinase in relation to carbohydrate utilization in muscles from marine invertebrates. Biochem. J. 1976, 160. [Google Scholar] [CrossRef] [Green Version]
- Alp, P.R.; Newsholme, E.A.; Zammit, V.A. Activities of citrate synthase and nad+-linked and nadp+-linked isocitrate dehydrogenase in muscle from vertebrates and invertebrates. Biochem. J. 1976, 154. [Google Scholar] [CrossRef] [PubMed]
- Guglielmo, C.G.; Haunerland, N.H.; Hochachka, P.W.; Williams, T.D. Seasonal dynamics of flight muscle fatty acid binding protein and catabolic enzymes in a migratory shorebird. Am. J. Physiol. 2002, 282, R1405–R1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCormick, S.D. Methods for nonlethal gill biopsy and measurement of na+, k+-atpase activity. Can. J. Fish. Aquat. Sci. 1993, 50, 656–658. [Google Scholar] [CrossRef]






| Hexokinase | Pyruvate Kinase | Lactate Dehydrogenase | Carnitine Palmitoyl Transferase | 3-Hydroxyacyl CoA Dehydrogenase | Citrate Synthase | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normoxia | Hypoxia | Normoxia | Hypoxia | Normoxia | Hypoxia | Normoxia | Hypoxia | Normoxia | Hypoxia | Normoxia | Hypoxia | |
| Brain | 16.64 ± 0.68 | 14.58 * ± 0.37 | 39.42 ± 3.06 | 37.75 ± 3.65 | 212.5 ± 13.23 | 204.12 ± 14.15 | 0.17 ± 0.01 | 0.14 * ± 0.04 | 0.1 ± 0.01 | 0.17 * ± 0.03 | 0.81 ± 0.24 | 0.57 ± 0.35 |
| Liver | 2.19 ± 0.23 | 1.89 ± 0.17 | 124.03 ± 20.95 | 65.15 * ± 14.38 | 344.89 ± 45.04 | 388.96 ± 43.38 | 10.34 ± 1.05 | 10.35 ± 1.04 | 0.29 ± 0.029 | 0.3 ± 0.03 | 2.09 ± 0.19 | 2.83 ± 0.42 |
| White muscle | 1.48 ± 0.11 | 2.69 ** ± 0.29 | 109.53 ± 5.2 | 96.63 ± 6.58 | 96.86 ± 13.51 | 118.53 ± 20.51 | 14.31 ± 1.67 | 11.73 ± 1.39 | 0.29 ± 0.05 | 0.46 ± 0.13 | 5.06 ± 0.31 | 4.71 ± 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farhat, E.; Cheng, H.; Romestaing, C.; Pamenter, M.; Weber, J.-M. Goldfish Response to Chronic Hypoxia: Mitochondrial Respiration, Fuel Preference and Energy Metabolism. Metabolites 2021, 11, 187. https://doi.org/10.3390/metabo11030187
Farhat E, Cheng H, Romestaing C, Pamenter M, Weber J-M. Goldfish Response to Chronic Hypoxia: Mitochondrial Respiration, Fuel Preference and Energy Metabolism. Metabolites. 2021; 11(3):187. https://doi.org/10.3390/metabo11030187
Chicago/Turabian StyleFarhat, Elie, Hang Cheng, Caroline Romestaing, Matthew Pamenter, and Jean-Michel Weber. 2021. "Goldfish Response to Chronic Hypoxia: Mitochondrial Respiration, Fuel Preference and Energy Metabolism" Metabolites 11, no. 3: 187. https://doi.org/10.3390/metabo11030187
APA StyleFarhat, E., Cheng, H., Romestaing, C., Pamenter, M., & Weber, J. -M. (2021). Goldfish Response to Chronic Hypoxia: Mitochondrial Respiration, Fuel Preference and Energy Metabolism. Metabolites, 11(3), 187. https://doi.org/10.3390/metabo11030187