A Time-Course Comparison of Skeletal Muscle Metabolomic Alterations in Walker-256 Tumour-Bearing Rats at Different Stages of Life
Abstract
:1. Introduction
2. Results
2.1. Walker-256 Tumour Growth Induced Cachexia, Leading to Loss of Body Weight, Spoliation of the Gastrocnemius Muscle, and Nitrogen Imbalance at Both Ages
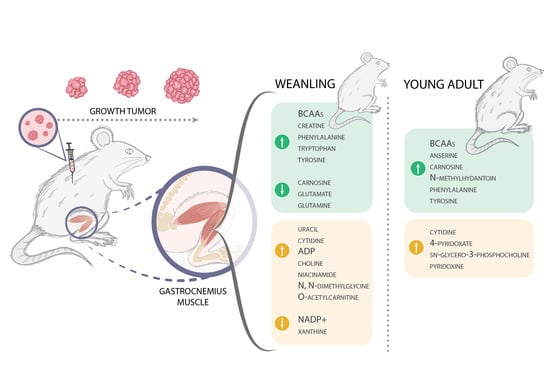
2.2. Cachexia Induces a Disorder in the Gastrocnemius Muscle’s Metabolomic Profile in Weanling and Young Adult Rats
2.2.1. Metabolites Are Affected throughout Tumour Evolution in Weanling and Young Adult Tumour-Bearing Animals
2.2.2. Metabolites Affected Regarding the Age of the Tumour-Bearing Animals
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Protocol
4.3. Nitrogen Balance
4.4. Metabolomic Analyses
4.4.1. Muscle Sample Preparation for 1HNMR Acquisition
4.4.2. 1H-NMR Spectra Acquisition and Metabolic Quantification
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, W.J.; Morley, J.E.; Argiles, J.M.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef]
- Fearon, K.C.; Glass, D.J.; Guttridge, D.C. Cancer Cachexia: Mediators, Signaling, and Metabolic Pathways. Cell Metab. 2012, 16, 153–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argiles, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Argiles, J.M.; Stemmler, B.; López-Soriano, F.J.; Busquets, S. Inter-tissue communication in cancer cachexia. Nat. Rev. Endocrinol. 2019, 15, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-associated cachexia. Nat. Rev. Dis. Prim. 2018, 4, 17105. [Google Scholar] [CrossRef]
- Penet, M.-F.; Bhujwalla, Z.M. Cancer Cachexia, Recent Advances, and Future Directions. Cancer J. 2015, 21, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Acharyya, S.; Ladner, K.J.; Nelsen, L.L.; Damrauer, J.; Reiser, P.J.; Swoap, S.; Guttridge, D.C. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J. Clin. Investig. 2004, 114, 370–378. [Google Scholar] [CrossRef]
- Der-Torossian, H.; Wysong, A.; Shadfar, S.; Willis, M.S.; McDunn, J.; Couch, M.E. Metabolic derangements in the gastrocnemius and the effect of Compound A therapy in a murine model of cancer cachexia. J. Cachex Sarcopenia Muscle 2013, 4, 145–155. [Google Scholar] [CrossRef]
- Vaitheesvaran, B.; Xu, J.; Yee, J.; Lu, Q.-Y.; Go, V.L.; Xiao, G.G.; Lee, W.-N. The Warburg effect: A balance of flux analysis. Metabolomics 2015, 11, 787–796. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.G.; Buel, G.R.; Blenis, J. Nutrient regulation of the mTOR Complex 1 signaling pathway. Mol. Cells 2013, 35, 463–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, I.; Jacobi, C.; Tardif, N.; Rooyackers, O.; Fearon, K. Omics/systems biology and cancer cachexia. Semin. Cell Dev. Biol. 2016, 54, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Springer, J.; Anker, S.D. The underestimated impact of cachexia. Expert Opin. Drug Discov. 2009, 5, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Von Haehling, S.; Anker, S.D. Cachexia as a major underestimated and unmet medical need: Facts and numbers. J. Cachex Sarcopenia Muscle 2010, 1, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.-J.; Zhao, J.-R.; Hao, J.; Li, B.; Huo, Y.; Han, Y.-L.; Wan, L.-L.; Li, J.; Huang, J.; Lu, J.; et al. Serum and urine metabolomics study reveals a distinct diagnostic model for cancer cachexia. J. Cachex Sarcopenia Muscle 2017, 9, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Viana, L.R.; Lopes-Aguiar, L.; Rosolen, R.R.; Dos Santos, R.W.; Gomes-Marcondes, M.C.C. 1H-NMR Based Serum Metabolomics Identifies Different Profile between Sarcopenia and Cancer Cachexia in Ageing Walker 256 Tumour-Bearing Rats. Metabolites 2020, 10, 161. [Google Scholar] [CrossRef] [Green Version]
- Viana, L.R.; Canevarolo, R.; Luiz, A.C.P.; Soares, R.F.; Lubaczeuski, C.; Zeri, A.C.D.M.; Gomes-Marcondes, M.C.C. Leucine-rich diet alters the 1H-NMR based metabolomic profile without changing the Walker-256 tumour mass in rats. BMC Cancer 2016, 16, 764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Twelkmeyer, B.; Tardif, N.; Rooyackers, O. Omics and cachexia. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 181–185. [Google Scholar] [CrossRef]
- Giskeødegård, G.F.; Madssen, T.S.; Euceda, L.R.; Tessem, M.; Moestue, S.A.; Bathen, T.F. NMR-based metabolomics of biofluids in cancer. NMR Biomed. 2019, 32, e3927. [Google Scholar] [CrossRef]
- QuanJun, Y.; Genjin, Y.; Lili, W.; Yan, H.; Yonglong, H.; Jin, L.; Jie, L.; Jinlu, H.; Cheng, G. Integrated analysis of serum and intact muscle metabonomics identify metabolic profiles of cancer cachexia in a dynamic mouse model. RSC Adv. 2015, 5, 92438–92448. [Google Scholar] [CrossRef]
- Schiessel, D.; Yamazaki, R.K.; Kryczyk, M.; Coelho, I.; Yamaguchi, A.A.; Pequito, D.C.T.; Brito, G.A.P.; Borghetti, G.; Fernandes, L.C. α-Linolenic Fatty Acid Supplementation Decreases Tumor Growth and Cachexia Parameters in Walker 256 Tumor-Bearing Rats. Nutr. Cancer 2015, 67, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Martins, H.A.; Bazotte, R.B.; Vicentini, G.E.; Lima, M.M.; Guarnier, F.A.; Hermes-Uliana, C.; Frez, F.C.V.; Bossolani, G.D.P.; Fracaro, L.; Fávaro, L.D.S.; et al. l-Glutamine supplementation promotes an improved energetic balance in Walker-256 tumor–bearing rats. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rischiteli, A.B.S.; Neto, N.I.P.; Gascho, K.; Carnier, M.; De Miranda, D.A.; Silva, F.P.; Boldarine, V.T.; Seelaender, M.; Ribeiro, E.B.; Oyama, L.M.; et al. A diet including xanthan gum triggers a pro-inflammatory response in Wistar rats inoculated with Walker 256 cells. PLoS ONE 2019, 14, e0218567. [Google Scholar] [CrossRef]
- Jackson, S.J.; Andrews, N.; Ball, D.; Bellantuono, I.; Gray, J.; Hachoumi, L.; Holmes, A.; Latcham, J.; Petrie, A.; Potter, P.; et al. Does age matter? The impact of rodent age on study outcomes. Lab. Anim. 2017, 51, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Agoston, D.V. How to Translate Time? The Temporal Aspect of Human and Rodent Biology. Front. Neurol. 2017, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Reid, J.; McKeaveney, C.; Martin, P. Communicating with Adolescents and Young Adults about Cancer-Associated Weight Loss. Curr. Oncol. Rep. 2019, 21, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Quintilhano, D.L.; Miksza, D.R.; Galia, W.B.D.S.; Ramalho, M.O.R.C.; Lucena, C.F.; Valle, M.M.R.; Graciano, M.F.R.; De Souza, H.M.; Bertolini, G.L. Insulin secretion decline in Walker-256 tumor-bearing rats is early, follows the course of cachexia, and is not improved by lixisenatide. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 394, 1–9. [Google Scholar] [CrossRef]
- Cruz, B.; Oliveira, A.; Gomes-Marcondes, M.C.C. L-leucine dietary supplementation modulates muscle protein degradation and increases pro-inflammatory cytokines in tumour-bearing rats. Cytokine 2017, 96, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Miyaguti, N.A.D.S.; De Oliveira, S.C.P.; Gomes-Marcondes, M.C.C. Maternal Leucine-Rich Diet Minimises Muscle Mass Loss in Tumour-bearing Adult Rat Offspring by Improving the Balance of Muscle Protein Synthesis and Degradation. Biomolecules 2019, 9, 229. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, S.P.; Santos, J.M.O.; Silva, M.P.C.E.; da Costa, R.M.G.; Medeiros, R. Cancer cachexia and its pathophysiology: Links with sarcopenia, anorexia and asthenia. J. Cachex Sarcopenia Muscle 2020, 11, 619–635. [Google Scholar] [CrossRef]
- Porporato, P. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis 2016, 5, e200. [Google Scholar] [CrossRef] [Green Version]
- Rausch, V.; Sala, V.; Penna, F.; Porporato, P.E.; Ghigo, A. Understanding the common mechanisms of heart and skeletal muscle wasting in cancer cachexia. Oncogenesis 2021, 10, 1–13. [Google Scholar] [CrossRef]
- Baracos, V.E. Regulation of skeletal-muscle–protein turnover in cancer-associated cachexia. Nutrition 2000, 16, 1015–1018. [Google Scholar] [CrossRef]
- Esper, D.H.; Harb, W.A. The Cancer Cachexia Syndrome: A Review of Metabolic and Clinical Manifestations. Nutr. Clin. Pr. 2005, 20, 369–376. [Google Scholar] [CrossRef]
- Tseng, Y.-C.; Kulp, S.K.; Lai, I.-L.; Hsu, E.-C.; He, W.A.; Frankhouser, D.E.; Yan, P.S.; Mo, X.; Bloomston, M.; Lesinski, G.B.; et al. Preclinical Investigation of the Novel Histone Deacetylase Inhibitor AR-42 in the Treatment of Cancer-Induced Cachexia. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef] [Green Version]
- Cui, P.; Shao, W.; Huang, C.; Wu, C.-J.; Jiang, B.; Lin, D. Metabolic derangements of skeletal muscle from a murine model of glioma cachexia. Skelet. Muscle 2019, 9, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baracos, V.E.; Mackenzie, M. Investigations of Branched-Chain Amino Acids and Their Metabolites in Animal Models of Cancer. J. Nutr. 2006, 136, 237S–242S. [Google Scholar] [CrossRef] [PubMed]
- Lautaoja, J.H.; Lalowski, M.; Nissinen, T.A.; Hentilä, J.; Shi, Y.; Ritvos, O.; Cheng, S.; Hulmi, J.J. Muscle and serum metabolomes are dysregulated in colon-26 tumor-bearing mice despite amelioration of cachexia with activin receptor type 2B ligand blockade. Am. J. Physiol. Metab. 2019, 316, E852–E865. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Huang, C.; Guo, J.; Wang, Q.; Liu, Z.; Zhuo, H.; Lin, D. Metabolic Profiling of Tumors, Sera, and Skeletal Muscles from an Orthotopic Murine Model of Gastric Cancer Associated-Cachexia. J. Proteome Res. 2019, 18, 1880–1892. [Google Scholar] [CrossRef]
- Aranibar, N.; Vassallo, J.D.; Rathmacher, J.; Stryker, S.; Zhang, Y.; Dai, J.; Janovitz, E.B.; Robertson, D.; Reily, M.; Lowe-Krentz, L.; et al. Identification of 1- and 3-methylhistidine as biomarkers of skeletal muscle toxicity by nuclear magnetic resonance-based metabolic profiling. Anal. Biochem. 2011, 410, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, P.; Jiang, J. Measurement of 1- and 3-methylhistidine in human urine by ultra performance liquid chromatography–tandem mass spectrometry. Clin. Chim. Acta 2012, 413, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Rodado, V.; Luque-Baena, R.; Vruchte, D.; Probert, F.; Lachmann, R.; Hendriksz, C.; Wraith, J.; Imrie, J.; Elizondo, D.; Sillence, D.; et al. 1H NMR-Linked Urinary Metabolic Profiling of Niemann-Pick Class C1 (NPC1) Disease: Identification of Potential New Biomarkers using Correlated Component Regression (CCR) and Genetic Algorithm (GA) Analysis Strategies. Curr. Metab. 2014, 2, 88–121. [Google Scholar] [CrossRef]
- Munro, H.N. Adaptation of body protein metabolism in adult and aging man. Clin. Nutr. 1982, 1, 95–108. [Google Scholar] [CrossRef]
- Vesali, R.; Klaude, M.; Thunblad, L.; Rooyackers, O.; Wernerman, J. Contractile protein breakdown in human leg skeletal muscle as estimated by [2H3]-3-methylhistidine: A new method. Metabolism 2004, 53, 1076–1080. [Google Scholar] [CrossRef]
- Cruz, B.; Oliveira, A.; Viana, L.; Lopes-Aguiar, L.; Canevarolo, R.; Colombera, M.; Valentim, R.; Garcia-Fóssa, F.; De Sousa, L.; Castelucci, B.; et al. Leucine-Rich Diet Modulates the Metabolomic and Proteomic Profile of Skeletal Muscle during Cancer Cachexia. Cancers 2020, 12, 1880. [Google Scholar] [CrossRef]
- Ubhi, B.K.; Riley, J.H.; Shaw, P.A.; Lomas, D.A.; Tal-Singer, R.; MacNee, W.; Griffin, J.L.; Connor, S.C. Metabolic profiling detects biomarkers of protein degradation in COPD patients. Eur. Respir. J. 2011, 40, 345–355. [Google Scholar] [CrossRef] [Green Version]
- Segaud, F.; Combaret, L.; Neveux, N.; Attaix, D.; Cynober, L.; Moinard, C. Effects of ornithine α-ketoglutarate on protein metabolism in Yoshida sarcoma-bearing rats. Clin. Nutr. 2007, 26, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Thaden, J.J.; Have, G.A.T.; Walker, D.K.; Engelen, M.P. Metabolic phenotyping using kinetic measurements in young and older healthy adults. Metabolism 2018, 78, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, T.; Kikuchi, N.; Ito, Y.; Yoshizawa, F.; Nishizawa, N. Suppression of Myofibrillar Protein Degradation after Refeeding in Young and Adult Mice. J. Nutr. Sci. Vitaminol. 2004, 50, 227–230. [Google Scholar] [CrossRef] [Green Version]
- Svanberg, E.; Möller-Loswick, A.-C.; Matthews, D.E.; Körner, U.; Lundholm, K. The effect of glutamine on protein balance and amino acid flux across arm and leg tissues in healthy volunteers. Clin. Physiol. 2001, 21, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Low, S.Y.; Taylor, P.M.; Ahmed, A.; I Pogson, C.; Rennie, M.J. Substrate-specificity of glutamine transporters in membrane vesicles from rat liver and skeletal muscle investigated using amino acid analogues. Biochem. J. 1991, 278, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Tapiero, H.; Mathé, G.; Couvreur, P.; Tew, K.D., II. Glutamine and glutamate. Biomed. Pharmacother 2002, 56, 446–457. [Google Scholar] [CrossRef]
- Rogers, K.C.; Söll, D. Divergence of glutamate and glutamine aminoacylation pathways: Providing the evolutionary rationale for mischarging. J. Mol. Evol. 1995, 40, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Artioli, G.G.; Sale, C.; Jones, R.L. Carnosine in health and disease. Eur. J. Sport Sci. 2018, 19, 30–39. [Google Scholar] [CrossRef]
- Rohm, M.; Zeigerer, A.; Machado, J.; Herzig, S. Energy metabolism in cachexia. EMBO Rep. 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Pin, F.; Barreto, R.; Couch, M.E.; Bonetto, A.; O’Connell, T.M. Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J. Cachex Sarcopenia Muscle 2019, 10, 140–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akram, M. Citric Acid Cycle and Role of its Intermediates in Metabolism. Cell Biophys. 2014, 68, 475–478. [Google Scholar] [CrossRef]
- Coles, C.A. Adipokines in healthy skeletal muscle and metabolic disease. In Growth Factors and Cytokines in Skeletal Muscle Development, Growth, Regeneration and Diasese; White, J., Smythe, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 133–160. ISBN 978-3-319-27509-3. [Google Scholar]
- Bowman, C.E.; Wolfgang, M.J. Role of the malonyl-CoA synthetase ACSF3 in mitochondrial metabolism. Adv. Biol. Regul. 2019, 71, 34–40. [Google Scholar] [CrossRef]
- Abdel-Aleem, S.; Nada, M.A.; Sayed-Ahmed, M.; Hendrickson, S.C.; Louis, J.S.; Walthall, H.P.; Lowe, J.E. Regulation of Fatty Acid Oxidation by Acetyl-CoA Generated from Glucose Utilization in Isolated Myocytes. J. Mol. Cell. Cardiol. 1996, 28, 825–833. [Google Scholar] [CrossRef]
- Reeds, P.J.; Berthold, H.K.; Boza, J.J.; Burrin, D.G.; Jahoor, F.; Jaksic, T.; Klein, P.D.; Keshen, T.; Miller, R.; Stoll, B.; et al. Inte-gration of amino acids and carbon intermediary metabolism: Studies with uniformly labeled tracers and mass isotopomer analysis. Eur. J. Pediatr. 1997, 156, S50–S58. [Google Scholar] [CrossRef]
- Kamei, Y.; Hatazawa, Y.; Uchitomi, R.; Yoshimura, R.; Miura, S. Regulation of Skeletal Muscle Function by Amino Acids. Nutrients 2020, 12, 261. [Google Scholar] [CrossRef] [Green Version]
- Rabinowitz, J.D.; Enerbäck, S. Lactate: The ugly duckling of energy metabolism. Nat. Metab. 2020, 2, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, P. The Laboratory Rat: Relating Its Age With Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar] [PubMed]
- Albanese, A.; Orto, L. Protein and amino acids. In Newer Methods of Nutritional Biochemistry; Albanese, A., Ed.; Elsevier: New York, NY, USA, 1963; pp. 1–112. [Google Scholar]
- Le Belle, J.E.; Harris, N.G.; Williams, S.R.; Bhakoo, K.K. A comparison of cell and tissue extraction techniques using high-resolution1H-NMR spectroscopy. NMR Biomed. 2002, 15, 37–44. [Google Scholar] [CrossRef]





| Pathway | Metabolite | Weanling | Young Adult | Weanling vs. Young Adult | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tumour Evolution | Tumour Evolution | Age | ||||||||
| Match Status | Regulation | Adjusted p-Value * | Match Status | Regulation | Adjusted p-Value * | Match Status | Regulation | Adjusted p-Value * | ||
| Aminoacyl-tRNA biosynthesis | Glutamate | 8/48 | ↓ | <0.01 | 5/48 | ns | <0.01 | 5/48 | ↑ vs. ↓ | 0.01 |
| Glutamine | ↓ | ns | ↑ vs. ↓ | |||||||
| Glycine | ns | ns | ↑ vs. ↓ | |||||||
| Histidine | ns | ns | ↑ vs. ↓ | |||||||
| Isoleucine | ↑ | ↑ | ns | |||||||
| Leucine | ↑ | ↑ | ns | |||||||
| Phenylalanine | ↑ | ↑ | ns | |||||||
| Tryptophan | ↑ | ns | ↑ vs. ↓ | |||||||
| Tyrosine | ↑ | ↑ | ns | |||||||
| Valine | ↑ | ↑ | ns | |||||||
| Valine, leucine, and isoleucine biosynthesis | Isoleucine | 3/8 | ↑ | <0.01 | 3/8 | ↑ | <0.01 | 0/8 | ns | ne |
| Leucine | ↑ | ↑ | ns | |||||||
| Valine | ↑ | ↑ | ns | |||||||
| Phenylalanine, tyrosine, and tryptophan biosynthesis | Phenylalanine | 2/4 | ↑ | 0.02 | 2/4 | ↑ | 0.01 | 0/4 | ns | ne |
| Tyrosine | ↑ | ↑ | ns | |||||||
| Nitrogen metabolism | Glutamate | 2/6 | ↓ | 0.03 | 0/6 | ns | ne | 2/6 | ↑ vs. ↓ | 0.03 |
| Glutamine | ↓ | ns | ↑ vs. ↓ | |||||||
| D-glutamine and D-glutamate metabolism | Glutamate | 2/6 | ↓ | 0.03 | 0/6 | ns | ne | 2/6 | ↑ vs. ↓ | 0.03 |
| Glutamine | ↓ | ns | ↑ vs. ↓ | |||||||
| Vitamin B6 metabolism | 4-Pyridoxate | 0/9 | ns | ne | 2/9 | ↑ | 0.04 | 2/9 | ↓ vs. ↑ | 0.06 |
| Pyridoxine | ns | ↑ | ↓ vs. ↑ | |||||||
| Histidine metabolism | Anserine | 2/16 | ns | 0.11 | 2/16 | ↑ | 0.07 | 6/16 | ↓ vs. ↑ | <0.01 |
| Carnosine | ↓ | ↑ | ↓ vs. ↑ | |||||||
| Glutamate | ↓ | ns | ↑ vs. ↓ | |||||||
| Histamine | ns | ns | ↑ vs. ↓ | |||||||
| Histidine | ns | ns | ↑ vs. ↓ | |||||||
| π-Methylhistidine | ns | ns | ↑ vs. ↓ | |||||||
| β-Alanine metabolism | Anserine | 2/21 | ns | 0.166 | 2/21 | ↑ | 0.11 | 4/21 | ↓ vs. ↑ | 0.01 |
| Carnosine | ↓ | ↑ | ↓ vs. ↑ | |||||||
| Histidine | ns | ns | ↑ vs. ↓ | |||||||
| Uracil | ↑ | ns | ↑ vs. ↓ | |||||||
| Glyoxylate and dicarboxylate metabolism | Acetate | 2/32 | ns | 0.27 | 0/32 | ns | ne | 4/32 | ↑ vs. ↓ | 0.01 |
| Glutamate | ↓ | ns | ↑ vs. ↓ | |||||||
| Glutamine | ↓ | ns | ↑ vs. ↓ | |||||||
| Glycine | ns | ns | ↑ vs. ↓ | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiocchetti, G.d.M.e.; Lopes-Aguiar, L.; Miyaguti, N.A.d.S.; Viana, L.R.; Salgado, C.d.M.; Orvoën, O.O.; Florindo, D.; Santos, R.W.d.; Cintra Gomes-Marcondes, M.C. A Time-Course Comparison of Skeletal Muscle Metabolomic Alterations in Walker-256 Tumour-Bearing Rats at Different Stages of Life. Metabolites 2021, 11, 404. https://doi.org/10.3390/metabo11060404
Chiocchetti GdMe, Lopes-Aguiar L, Miyaguti NAdS, Viana LR, Salgado CdM, Orvoën OO, Florindo D, Santos RWd, Cintra Gomes-Marcondes MC. A Time-Course Comparison of Skeletal Muscle Metabolomic Alterations in Walker-256 Tumour-Bearing Rats at Different Stages of Life. Metabolites. 2021; 11(6):404. https://doi.org/10.3390/metabo11060404
Chicago/Turabian StyleChiocchetti, Gabriela de Matuoka e, Leisa Lopes-Aguiar, Natália Angelo da Silva Miyaguti, Lais Rosa Viana, Carla de Moraes Salgado, Ophelie Ocean Orvoën, Derly Florindo, Rogério Williams dos Santos, and Maria Cristina Cintra Gomes-Marcondes. 2021. "A Time-Course Comparison of Skeletal Muscle Metabolomic Alterations in Walker-256 Tumour-Bearing Rats at Different Stages of Life" Metabolites 11, no. 6: 404. https://doi.org/10.3390/metabo11060404
APA StyleChiocchetti, G. d. M. e., Lopes-Aguiar, L., Miyaguti, N. A. d. S., Viana, L. R., Salgado, C. d. M., Orvoën, O. O., Florindo, D., Santos, R. W. d., & Cintra Gomes-Marcondes, M. C. (2021). A Time-Course Comparison of Skeletal Muscle Metabolomic Alterations in Walker-256 Tumour-Bearing Rats at Different Stages of Life. Metabolites, 11(6), 404. https://doi.org/10.3390/metabo11060404