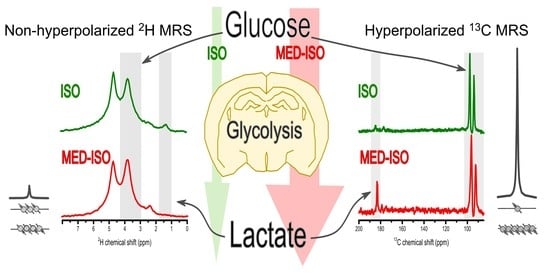
Measuring Glycolytic Activity with Hyperpolarized [2H7, U-13C6] D-Glucose in the Naive Mouse Brain under Different Anesthetic Conditions
Abstract
:1. Introduction
2. Results
2.1. Neurochemical Profile
2.2. Measurement of Glc Metabolism by [6,6′-2H2] Glc MRS
2.3. Real-Time Measurement of HP [2H7, U-13C6] Glc Metabolism
2.4. Numerical Simulation
3. Discussion
3.1. HP [2H7, U-13C6] Glc Is Sensitive to Different Metabolic Parameters Than Its Non-HP X-Nuclei MRS Counterparts
3.2. Brain Consciousness Influence De Novo Lac Production from HP [2H7, U-13C6] Glc and the Dynamics of 2H-Glx Labelling from Non-HP [6,6′-2H2] Glc
3.3. Anesthesia Has a Systemic Effect on Metabolism
3.4. Towards Quantitative Analysis of De Novo Lac Production from HP [2H7, U-13C6] Glc
4. Materials and Methods
4.1. Animal Experimentation
4.2. Study Design
4.3. Hyperpolarization
4.4. Animal Preparation for Magnetic Resonance Scans
4.5. MRS Measurements
4.5.1. In Vivo 1H Magnetic Resonance Spectroscopy
4.5.2. In Vivo HP 13C MRS
4.5.3. In Vivo Non-HP 2H MRS
4.6. Numerical Simulations
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mergenthaler, P.; Lindauer, U.; Dienel, G.A.; Meisel, A. Sugar for the brain: The role of glucose in physiological and pathological brain function. Trends Neurosci. 2013, 36, 587–597. [Google Scholar] [CrossRef] [Green Version]
- Pellerin, L. Food for thought: The importance of glucose and other energy substrates for sustaining brain function under varying levels of activity. Diabetes Metab. 2010, 36, S59–S63. [Google Scholar] [CrossRef]
- Sokoloff, L. Energetics of Functional Activation in Neural Tissues. Neurochem. Res. 1999, 24, 321–329. [Google Scholar] [CrossRef]
- Sonnay, S.; Gruetter, R.; Duarte, J.M.N. How Energy Metabolism Supports Cerebral Function: Insights from 13C Magnetic Resonance Studies In vivo. Front. Neurosci. 2017, 11, 288. [Google Scholar] [CrossRef] [Green Version]
- Ntziachristos, V.; Pleitez, M.A.; Aime, S.; Brindle, K.M. Emerging Technologies to Image Tissue Metabolism. Cell Metab. 2019, 29, 518–538. [Google Scholar] [CrossRef] [Green Version]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Duran-Aniotz, C.; Hetz, C. Glucose Metabolism: A Sweet Relief of Alzheimer’s Disease. Curr. Biol. 2016, 26, R806–R809. [Google Scholar] [CrossRef] [Green Version]
- Detka, J.; Kurek, A.; Kucharczyk, M.; Głombik, K.; Basta-Kaim, A.; Kubera, M.; Lason, W.; Budziszewska, B. Brain glucose metabolism in an animal model of depression. Neuroscience 2015, 295, 198–208. [Google Scholar] [CrossRef]
- Phelps, M.E.; Huang, S.C.; Hoffman, E.J.; Selin, C.; Sokoloff, L.; Kuhl, D.E. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: Validation of method. Ann. Neurol. 1979, 6, 371–388. [Google Scholar] [CrossRef]
- Huang, S.C.; Phelps, M.E.; Hoffman, E.J.; Sideris, K.; Selin, C.J.; Kuhl, D.E. Noninvasive determination of local cerebral metabolic rate of glucose in man. Am. J. Physiol. Metab. 1980, 238, E69–E82. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; McConathy, J. Overview of PET Tracers for Brain Tumor Imaging. PET Clin. 2013, 8, 129–146. [Google Scholar] [CrossRef]
- Ward, K.; Aletras, A.; Balaban, R. A New Class of Contrast Agents for MRI Based on Proton Chemical Exchange Dependent Saturation Transfer (CEST). J. Magn. Reson. 2000, 143, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.W.; McMahon, M.T.; Kato, Y.; Liu, G.; Bulte, J.W.M.; Bhujwalla, Z.M.; Artemov, D.; van Zijl, P.C.M. NaturalD-glucose as a biodegradable MRI contrast agent for detecting cancer. Magn. Reson. Med. 2012, 68, 1764–1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, T.; Mehrens, H.; Hendrich, K.S.; Kim, S.-G. Mapping Brain Glucose Uptake with Chemical Exchange-Sensitive Spin-Lock Magnetic Resonance Imaging. Br. J. Pharmacol. 2014, 34, 1402–1410. [Google Scholar] [CrossRef] [Green Version]
- Harris, R.J.; Cloughesy, T.F.; Liau, L.; Prins, R.M.; Antonios, J.P.; Li, D.; Yong, W.H.; Pope, W.B.; Lai, A.; Nghiemphu, P.L.; et al. pH-weighted molecular imaging of gliomas using amine chemical exchange saturation transfer MRI. Neuro Oncol. 2015, 17, 1514–1524. [Google Scholar] [CrossRef] [Green Version]
- Gruetter, R.; Seaquist, E.R.; Ugurbil, K. A mathematical model of compartmentalized neurotransmitter metabolism in the human brain. Am. J. Physiol. Metab. 2001, 281, E100–E112. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.; Zhu, X.-H.; Zhang, Y.; Mateescu, G.; Chen, W. Quantitative assessment of brain glucose metabolic rates using in vivo deuterium magnetic resonance spectroscopy. Br. J. Pharmacol. 2017, 37, 3518–3530. [Google Scholar] [CrossRef] [PubMed]
- De Feyter, H.M.; Behar, K.L.; Corbin, Z.A.; Fulbright, R.K.; Brown, P.B.; McIntyre, S.; Nixon, T.W.; Rothman, D.L.; De Graaf, R.A. Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Sci. Adv. 2018, 4, eaat7314. [Google Scholar] [CrossRef] [Green Version]
- Ardenkjaer-Larsen, J.H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M.H.; Servin, R.; Thaning, M.; Golman, K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. USA 2003, 100, 10158–10163. [Google Scholar] [CrossRef] [Green Version]
- Golman, K.; Olsson, L.E.; Axelsson, O.; Månsson, S.; Karlsson, M.; Petersson, J.S. Molecular imaging using hyperpolarized13C. Br. J. Radiol. 2003, 76, S118–S127. [Google Scholar] [CrossRef] [PubMed]
- Mishkovsky, M.; Comment, A. Hyperpolarized MRS: New tool to study real-time brain function and metabolism. Anal. Biochem. 2017, 529, 270–277. [Google Scholar] [CrossRef]
- Le Page, L.M.; Guglielmetti, C.; Taglang, C.; Chaumeil, M.M. Imaging Brain Metabolism Using Hyperpolarized 13C Magnetic Resonance Spectroscopy. Trends Neurosci. 2020, 43, 343–354. [Google Scholar] [CrossRef]
- Grist, J.T.; Miller, J.; Zaccagna, F.; McLean, M.A.; Riemer, F.; Matys, T.; Tyler, D.J.; Laustsen, C.; Coles, A.J.; Gallagher, F.A. Hyperpolarized 13C MRI: A novel approach for probing cerebral metabolism in health and neurological disease. Br. J. Pharmacol. 2020, 40, 1137–1147. [Google Scholar] [CrossRef]
- Kurhanewicz, J.; Vigneron, D.B.; Ardenkjaer-Larsen, J.H.; Bankson, J.A.; Brindle, K.; Cunningham, C.; Gallagher, F.A.; Keshari, K.R.; Kjaer, A.; Laustsen, C.; et al. Hyperpolarized 13C MRI: Path to Clinical Translation in Oncology. Neoplasia 2019, 21, 1–16. [Google Scholar] [CrossRef]
- Salamanca-Cardona, L.; Keshari, K.R. (13)C-labeled biochemical probes for the study of cancer metabolism with dynamic nuclear polarization-enhanced magnetic resonance imaging. Cancer Metab. 2015, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Allouche-Arnon, H.; Wade, T.; Waldner, L.F.; Miller, V.N.; Gomori, J.M.; Katz-Brull, R.; McKenzie, C.A. In vivomagnetic resonance imaging of glucose—initial experience. Contrast Media Mol. Imaging 2012, 8, 72–82. [Google Scholar] [CrossRef]
- Rodrigues, T.B.; Serrao, E.M.; Kennedy, B.W.; Hu, D.-E.; Kettunen, M.I.; Brindle, K.M. Magnetic resonance imaging of tumor glycolysis using hyperpolarized 13C-labeled glucose. Nat. Med. 2014, 20, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Mishkovsky, M.; Anderson, B.; Karlsson, M.; Lerche, M.H.; Sherry, A.D.; Gruetter, R.; Kovacs, Z.; Comment, A. Measuring glucose cerebral metabolism in the healthy mouse using hyperpolarized 13C magnetic resonance. Sci. Rep. 2017, 7, 11719. [Google Scholar] [CrossRef] [Green Version]
- Mishkovsky, M.; Gusyatiner, O.; Lanz, B.; Cudalbu, C.; Vassallo, I.; Hamou, M.-F.; Bloch, J.; Comment, A.; Gruetter, R.; Hegi, M.E. Hyperpolarized 13C-glucose magnetic resonance highlights reduced aerobic glycolysis in vivo in infiltrative glioblastoma. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Sibson, N.R.; Dhankhar, A.; Mason, G.F.; Rothman, D.L.; Behar, K.; Shulman, R.G. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc. Natl. Acad. Sci. USA 1998, 95, 316–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, I.-Y.; Lei, H.; Gruetter, R. Effect of Deep Pentobarbital Anesthesia on Neurotransmitter Metabolism in Vivo: On the Correlation of Total Glucose Consumption with Glutamatergic Action. Br. J. Pharmacol. 2002, 22, 1343–1351. [Google Scholar] [CrossRef] [Green Version]
- Conzen, P.F.; Vollmar, B.; Habazettl, H.; Frink, E.J.; Peter, K.; Messmer, K. Systemic and Regional Hemodynamics of Isoflurane and Sevoflurane in Rats. Anesth. Analg. 1992, 74, 79–88. [Google Scholar] [CrossRef]
- Todd, M.M.; Weeks, J. Comparative Effects of Propofol, Pentobarbital, and Isoflurane on Cerebral Blood Flow and Blood Volume. J. Neurosurg. Anesthesiol. 1996, 8, 296–303. [Google Scholar] [CrossRef]
- Paasonen, J.; Stenroos, P.; Salo, R.A.; Kiviniemi, V.; Gröhn, O. Functional connectivity under six anesthesia protocols and the awake condition in rat brain. NeuroImage 2018, 172, 9–20. [Google Scholar] [CrossRef]
- Baumgart, J.; Zhou, Z.-Y.; Hara, M.; Cook, D.C.; Hoppa, M.B.; Ryan, T.; Hemmings, H.C. Isoflurane inhibits synaptic vesicle exocytosis through reduced Ca2+ influx, not Ca2+-exocytosis coupling. Proc. Natl. Acad. Sci. USA 2015, 112, 11959–11964. [Google Scholar] [CrossRef] [Green Version]
- Hemmings, H.C.; Riegelhaupt, P.M.; Kelz, M.B.; Solt, K.; Eckenhoff, R.G.; Orser, B.A.; Goldstein, P.A. Towards a Comprehensive Understanding of Anesthetic Mechanisms of Action: A Decade of Discovery. Trends Pharmacol. Sci. 2019, 40, 464–481. [Google Scholar] [CrossRef]
- Grandjean, J.; Schroeter, A.; Batata, I.; Rudin, M. Optimization of anesthesia protocol for resting-state fMRI in mice based on differential effects of anesthetics on functional connectivity patterns. NeuroImage 2014, 102, 838–847. [Google Scholar] [CrossRef]
- Lanz, B.; Abaei, A.; Braissant, O.; Choi, I.; Cudalbu, C.; Henry, P.; Gruetter, R.; Kara, F.; Kantarci, K.; Lee, P.; et al. Magnetic resonance spectroscopy in the rodent brain: Experts’ consensus recommendations. NMR Biomed. 2021, 34, e4325. [Google Scholar] [CrossRef]
- Mahar, R.; Zeng, H.; Giacalone, A.; Ragavan, M.; Mareci, T.H.; Merritt, M.E. Deuterated water imaging of the rat brain following metabolism of [ 2 H 7 ]glucose. Magn. Reson. Med. 2021, 85, 3049–3059. [Google Scholar] [CrossRef]
- Lanz, B.; Gruetter, R.; Duarte, J.M.N. Metabolic Flux and Compartmentation Analysis in the Brain In vivo. Front. Endocrinol. 2013, 4, 156. [Google Scholar] [CrossRef] [Green Version]
- Lai, M.; Gruetter, R.; Lanz, B. Progress towards in vivo brain 13C-MRS in mice: Metabolic flux analysis in small tissue volumes. Anal. Biochem. 2017, 529, 229–244. [Google Scholar] [CrossRef] [Green Version]
- Webb, A. Increasing the Sensitivity of Magnetic Resonance Spectroscopy and Imaging. Anal. Chem. 2011, 84, 9–16. [Google Scholar] [CrossRef]
- Marjanska, M.; Shestov, A.A.; Deelchand, D.K.; Kittelson, E.; Henry, P.-G. Brain metabolism under different anesthetic conditions using hyperpolarized [1-13 C]pyruvate and [2-13 C]pyruvate. NMR Biomed. 2018, 31, e4012. [Google Scholar] [CrossRef]
- Andersen, N.B.; Amaranath, L. Anesthetic Effects on Transport Across Cell Membranes. Anesthesiology 1973, 39, 126–152. [Google Scholar] [CrossRef]
- Greene, N.M. Inhalation Anesthetics and Permeability of Human Erythrocytes to Monosaccharides. Anesthesiology 1965, 26, 731–742. [Google Scholar] [CrossRef]
- Greene, N.M.; Cervenko, F.W. Inhalation anesthetics, carbon dioxide and glucose transport across red cell membranes. Acta Anaesthesiol. Scand. 1967, 11, 3–18. [Google Scholar] [CrossRef]
- Ori, C.; Dam, M.; Pizzolato, G.; Battistin, L.; Giron, G. Effects of Isoflurane Anesthesia on Local Cerebral Glucose Utilization in the Rat. Anesthesiology 1986, 65, 152–156. [Google Scholar] [CrossRef]
- Kohro, S.; Hogan, Q.H.; Nakae, Y.; Yamakage, M.; Bosnjak, Z.J. Anesthetic Effects on Mitochondrial ATP-sensitive K Channel. Anesthesiology 2001, 95, 1435–1440. [Google Scholar] [CrossRef] [Green Version]
- Rottenberg, H. Uncoupling of oxidative phosphorylation in rat liver mitochondria by general anesthetics. Proc. Natl. Acad. Sci. USA 1983, 80, 3313–3317. [Google Scholar] [CrossRef] [Green Version]
- Toyama, H.; Ichise, M.; Liow, J.-S.; Vines, D.C.; Seneca, N.M.; Modell, K.J.; Seidel, J.; Green, M.V.; Innis, R.B. Evaluation of anesthesia effects on [18F]FDG uptake in mouse brain and heart using small animal PET. Nucl. Med. Biol. 2004, 31, 251–256. [Google Scholar] [CrossRef]
- Alkire, M.T.M.; Haier, R.J.P.; Shah, N.K.M.; Anderson, C.T.M. Positron Emission Tomography Study of Regional Cerebral Metabolism in Humans during Isoflurane Anesthesia. Anesthesiology 1997, 86, 549–557. [Google Scholar] [CrossRef] [Green Version]
- Kofke, W.A.; Hawkins, R.A.; Davis, D.W.; Biebuyck, J.F.; Miller, R.D. Comparison of the Effects of Volatile Anesthetics on Brain Glucose Metabolism in Rats. Anesthesiology 1987, 66, 810–813. [Google Scholar] [CrossRef]
- Lenz, C.; Rebel, A.; van Ackern, K.; Kuschinsky, W.; Waschke, K.F. Local Cerebral Blood Flow, Local Cerebral Glucose Utilization, and Flow-Metabolism Coupling during Sevoflurane versus Isoflurane Anesthesia in Rats. Anesthesiology 1998, 89, 1480–1488. [Google Scholar] [CrossRef]
- Masamoto, K.; Kanno, I. Anesthesia and the Quantitative Evaluation of Neurovascular Coupling. Br. J. Pharmacol. 2012, 32, 1233–1247. [Google Scholar] [CrossRef] [Green Version]
- Fox, P.; Raichle, M.E.; Mintun, M.A.; Dence, C. Nonoxidative glucose consumption during focal physiologic neural activity. Science 1988, 241, 462–464. [Google Scholar] [CrossRef]
- Prichard, J.; Rothman, D.; Novotny, E.; Petroff, O.; Kuwabara, T.; Avison, M.; Howseman, A.; Hanstock, C.; Shulman, R. Lactate rise detected by 1H NMR in human visual cortex during physiologic stimulation. Proc. Natl. Acad. Sci. USA 1991, 88, 5829–5831. [Google Scholar] [CrossRef] [Green Version]
- Hertz, L.; Schousboe, A. Energy metabolism in glutamatergic neurons, GABAergic neurons and astrocytes in primary cultures. Neurochem. Res. 1988, 13, 605–610. [Google Scholar] [CrossRef]
- Tsacopoulos, M.; Magistretti, P.J. Metabolic coupling between glia and neurons. J. Neurosci. 1996, 16, 877–885. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Sorg, O.; Yu, N.; Martin, J.-L.; Pellerin, L. Neurotransmitters Regulate Energy Metabolism in Astrocytes: Implications for the Metabolic Trafficking between Neural Cells. Dev. Neurosci. 1993, 15, 306–312. [Google Scholar] [CrossRef]
- Valette, J.; Guillermier, M.; Besret, L.; Hantraye, P.; Bloch, G.; Lebon, V. Isoflurane Strongly Affects the Diffusion of Intracellular Metabolites, as Shown by 1H Nuclear Magnetic Resonance Spectroscopy of the Monkey Brain. Br. J. Pharmacol. 2006, 27, 588–596. [Google Scholar] [CrossRef] [Green Version]
- Horn, T.; Klein, J. Lactate levels in the brain are elevated upon exposure to volatile anesthetics: A microdialysis study. Neurochem. Int. 2010, 57, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Durand, J.L.; Hosinking, W.; Jelicks, L.A. Time Course of Effects of Inhalation Anesthesia on Blood Glucose Level in Male and Female C57BL/6 Mice. Horm. Metab. Res. 2009, 41, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Boretius, S.; Tammer, R.; Michaelis, T.; Brockmöller, J.; Frahm, J. Halogenated volatile anesthetics alter brain metabolism as revealed by proton magnetic resonance spectroscopy of mice in vivo. NeuroImage 2013, 69, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Michenfelder, J.D.; Theye, R.A. In vivo toxic effects of halothane on canine cerebral metabolic pathways. Am. J. Physiol. Content 1975, 229, 1050–1055. [Google Scholar] [CrossRef]
- Schwarzkopf, T.M.; Horn, T.; Lang, D.; Klein, J. Blood gases and energy metabolites in mouse blood before and after cerebral ischemia: The effects of anesthetics. Exp. Biol. Med. 2013, 238, 84–89. [Google Scholar] [CrossRef]
- Muravchick, S.; Levy, R.J.; Warltier, D.C. Clinical Implications of Mitochondrial Dysfunction. Anesthesiology 2006, 105, 819–837. [Google Scholar] [CrossRef] [Green Version]
- Sirmpilatze, N.; Baudewig, J.; Boretius, S. Temporal stability of fMRI in medetomidine-anesthetized rats. Sci. Rep. 2019, 9, 16673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capozzi, A.; Patel, S.; Wenckebach, W.T.; Karlsson, M.; Lerche, M.H.; Ardenkjær-Larsen, J.H.; Wenckebach, T.; Ardenkjaer-Larsen, J.H. Gadolinium Effect at High-Magnetic-Field DNP: 70% 13C Polarization of [U-13C] Glucose Using Trityl. J. Phys. Chem. Lett. 2019, 10, 3420–3425. [Google Scholar] [CrossRef]
- Cheng, T.; Capozzi, A.; Takado, Y.; Balzan, R.; Comment, A. Over 35% liquid-state 13C polarization obtained via dissolution dynamic nuclear polarization at 7 T and 1 K using ubiquitous nitroxyl radicals. Phys. Chem. Chem. Phys. 2013, 15, 20819. [Google Scholar] [CrossRef] [PubMed]
- Comment, A.; Brandt, B.V.D.; Uffmann, K.; Kurdzesau, F.; Jannin, S.; Konter, J.; Hautle, P.; Wenckebach, W.; Gruetter, R.; Van Der Klink, J. Design and performance of a DNP prepolarizer coupled to a rodent MRI scanner. Concepts Magn. Reson. Part B Magn. Reson. Eng. 2007, 31, 255–269. [Google Scholar] [CrossRef] [Green Version]
- Cheng, T.; Mishkovsky, M.; Bastiaansen, J.A.; Ouari, O.; Hautle, P.; Tordo, P.; Brandt, B.V.D.; Comment, A. Automated transfer and injection of hyperpolarized molecules with polarization measurement prior to in vivo NMR. NMR Biomed. 2013, 26, 1582–1588. [Google Scholar] [CrossRef]
- Reynaud, O.; da Silva, A.R.; Gruetter, R.; Jelescu, I.O. Multi-slice passband bSSFP for human and rodent fMRI at ultra-high field. J. Magn. Reson. 2019, 305, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Gruetter, R.; Tkac, I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn. Reson. Med. 2000, 43, 319–323. [Google Scholar] [CrossRef] [Green Version]
- Mlynarik, V.; Gambarota, G.; Frenkel, H.; Gruetter, R. Localized short-echo-time proton MR spectroscopy with full signal-intensity acquisition. Magn. Reson. Med. 2006, 56, 965–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provencher, S.W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993, 30, 672–679. [Google Scholar] [CrossRef]
- Nagy, C.; Einwallner, E. Study of In Vivo Glucose Metabolism in High-fat Diet-fed Mice Using Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT). J. Vis. Exp. 2018, e56672. [Google Scholar] [CrossRef] [PubMed]
- Staewen, R.S.; Johnson, A.J.; Ross, B.D.; Parrish, T.; Merkle, H.; Garwood, M. 3-D FLASH Imaging Using a Single Surface Coil and a New Adiabatic Pulse, BIR-4. Investig. Radiol. 1990, 25, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Naressi, A.; Couturier, C.; Devos, J.M.; Janssen, M.; Mangeat, C.; De Beer, R.; Graveron-Demilly, D. Java-based graphical user interface for the MRUI quantitation package. Magma Magn. Reson. Mater. Phys. Biol. Med. 2001, 12, 141–152. [Google Scholar] [CrossRef]
- Leong, S.F.; Lai, J.C.K.; Lim, L.; Clark, J.B. Energy-Metabolising Enzymes in Brain Regions of Adult and Aging Rats. J. Neurochem. 1981, 37, 1548–1556. [Google Scholar] [CrossRef]
- Xu, S.; Yang, J.; Shen, J. In vivo13C saturation transfer effect of the lactate dehydrogenase reaction. Magn. Reson. Med. 2007, 57, 258–264. [Google Scholar] [CrossRef]
- Lai, M.; Lanz, B.; Poitry-Yamate, C.; Romero, J.F.; Berset, C.; Cudalbu, C.; Gruetter, R. In vivo 13C MRS in the mouse brain at 14.1 Tesla and metabolic flux quantification under infusion of [1,6-13C2]glucose. Br. J. Pharmacol. 2017, 38, 1701–1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, C.E.; Karlsson, M.; Winther, J.R.; Jensen, P.R.; Lerche, M.H. Non-invasive In-cell Determination of Free Cytosolic [NAD+]/[NADH] Ratios Using Hyperpolarized Glucose Show Large Variations in Metabolic Phenotypes. J. Biol. Chem. 2014, 289, 2344–2352. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flatt, E.; Lanz, B.; Pilloud, Y.; Capozzi, A.; Lerche, M.H.; Gruetter, R.; Mishkovsky, M. Measuring Glycolytic Activity with Hyperpolarized [2H7, U-13C6] D-Glucose in the Naive Mouse Brain under Different Anesthetic Conditions. Metabolites 2021, 11, 413. https://doi.org/10.3390/metabo11070413
Flatt E, Lanz B, Pilloud Y, Capozzi A, Lerche MH, Gruetter R, Mishkovsky M. Measuring Glycolytic Activity with Hyperpolarized [2H7, U-13C6] D-Glucose in the Naive Mouse Brain under Different Anesthetic Conditions. Metabolites. 2021; 11(7):413. https://doi.org/10.3390/metabo11070413
Chicago/Turabian StyleFlatt, Emmanuelle, Bernard Lanz, Yves Pilloud, Andrea Capozzi, Mathilde Hauge Lerche, Rolf Gruetter, and Mor Mishkovsky. 2021. "Measuring Glycolytic Activity with Hyperpolarized [2H7, U-13C6] D-Glucose in the Naive Mouse Brain under Different Anesthetic Conditions" Metabolites 11, no. 7: 413. https://doi.org/10.3390/metabo11070413
APA StyleFlatt, E., Lanz, B., Pilloud, Y., Capozzi, A., Lerche, M. H., Gruetter, R., & Mishkovsky, M. (2021). Measuring Glycolytic Activity with Hyperpolarized [2H7, U-13C6] D-Glucose in the Naive Mouse Brain under Different Anesthetic Conditions. Metabolites, 11(7), 413. https://doi.org/10.3390/metabo11070413