Uncoupling Thermotolerance and Growth Performance in Chinook Salmon: Blood Biochemistry and Immune Capacity
Abstract
:1. Introduction
2. Results and Discussion
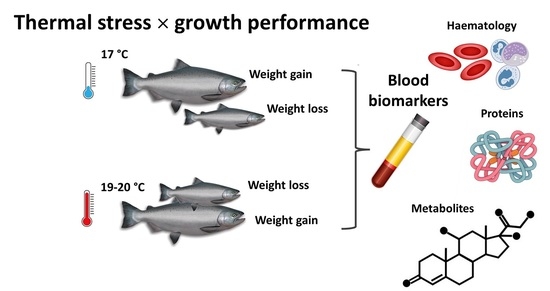
2.1. Survival and Growth
2.2. Thermal Stress Associations
2.3. Growth Performance Associations
2.4. Overview and Future Directions
3. Material and Methods
3.1. Experimental Design and Sampling
3.2. Biometrics
3.3. Cellular-Based Parameters
3.4. Plasma Biochemistry Parameters
3.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. FishStatJ: Universal Software for Fishery Statistical Time Series; Food and Agriculture Organization: Rome, Italy, 2021. [Google Scholar]
- Law, C.S.; Rickard, G.J.; Mikaloff-Fletcher, S.E.; Pinkerton, M.H.; Behrens, E.; Chiswell, S.M.; Currie, K. Climate change projections for the surface ocean around New Zealand. N. Z. J. Mar. Freshw. Res. 2018, 52, 309–335. [Google Scholar] [CrossRef]
- Sutton, P.J.; Bowen, M. Ocean temperature change around New Zealand over the last 36 years. N. Z. J. Mar. Freshw. Res. 2019, 53, 305–326. [Google Scholar] [CrossRef]
- Salinger, M.J.; Diamond, H.J.; Behrens, E.; Fernandez, D.; Fitzharris, B.B.; Herold, N.; Johnstone, P.; Kerckhoffs, H.; Mullan, A.B.; Parker, A.K.; et al. Unparalleled coupled ocean-atmosphere summer heatwaves in the New Zealand region: Drivers, mechanisms and impacts. Clim. Chang. 2020, 162, 485–506. [Google Scholar] [CrossRef]
- Salinger, M.J.; Renwick, J.; Behrens, E.; Mullan, A.B.; Diamond, H.J.; Sirguey, P.; Smith, R.O.; Trought, M.C.; Cullen, N.J.; Fitzharris, B.B.; et al. The unprecedented coupled ocean-atmosphere summer heatwave in the New Zealand region 2017/18: Drivers, mechanisms and impacts. Environ. Res. Lett. 2019, 14, 044023. [Google Scholar] [CrossRef]
- Broekhuizen, N.; Plew, D.R.; Pinkerton, M.H.; Gall, M.G. Sea temperature rise over the period 2002–2020 in Pelorus Sound, New Zealand–with possible implications for the aquaculture industry. N. Z. J. Mar. Freshw. Res. 2021, 55, 46–64. [Google Scholar] [CrossRef]
- Richter, A.; Kolmes, S.A. Maximum temperature limits for Chinook, coho, and chum salmon, and steelhead trout in the Pacific Northwest. Rev. Fish. Sci. 2005, 13, 23–49. [Google Scholar] [CrossRef]
- NZKS. Annual Report FY19; New Zealand King Salmon Ltd.: Nelson, New Zealand, 2019. [Google Scholar]
- Chiswell, S.M.; Sutton, P.J. Relationships between long-term ocean warming, marine heat waves and primary production in the New Zealand region. N. Z. J. Mar. Freshw. Res. 2020, 54, 614–635. [Google Scholar] [CrossRef]
- Myrick, C.A.; Cech, J.J. Temperature Effects on Chinook Salmon and Steelhead: A Review Focusing on California’s Central Valley Populations; Technical Publication 01-1; Bay-Delta Modeling Forum: Davis, CA, USA, 2001. [Google Scholar]
- Whitney, J.E.; Al-Chokhachy, R.; Bunnell, D.B.; Caldwell, C.A.; Cooke, S.J.; Eliason, E.J.; Rogers, M.; Lynch, A.J.; Paukert, C.P. Physiological basis of climate change impacts on North American inland fishes. Fisheries 2016, 41, 332–345. [Google Scholar] [CrossRef]
- Wade, N.M.; Clark, T.D.; Maynard, B.T.; Atherton, S.; Wilkinson, R.J.; Smullen, R.P.; Taylor, R.S. Effects of an unprecedented summer heatwave on the growth performance, flesh colour and plasma biochemistry of marine cage-farmed Atlantic salmon (Salmo salar). J. Therm. Biol. 2019, 80, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2020, 98, 1496–1508. [Google Scholar] [CrossRef] [PubMed]
- Rebl, A.; Korytář, T.; Borchel, A.; Bochert, R.; Strzelczyk, J.E.; Goldammer, T.; Verleih, M. The synergistic interaction of thermal stress coupled with overstocking strongly modulates the transcriptomic activity and immune capacity of rainbow trout (Oncorhynchus mykiss). Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- Montero, D.; Lalumera, G.; Izquierdo, M.S.; Caballero, M.J.; Saroglia, M.; Tort, L. Establishment of dominance relationships in gilthead sea bream Sparus aurata juveniles during feeding: Effects on feeding behaviour, feed utilization and fish health. J. Fish Biol. 2009, 74, 790–805. [Google Scholar] [CrossRef] [PubMed]
- Unrein, J.R. Early Self-Sorting Behavior in Chinook Salmon is Correlated with Variation in Growth, Behavior and Morphology Later in Life. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 2014. [Google Scholar]
- Damsgård, B.; Evensen, T.H.; Øverli, Ø.; Gorissen, M.; Ebbesson, L.O.; Rey, S.; Höglund, E. Proactive avoidance behaviour and pace-of-life syndrome in Atlantic salmon. R. Soc. Open Sci. 2019, 6, 181859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamaki, K.; Satou, Y. Caspases: Evolutionary aspects of their functions in vertebrates. J. Fish Biol. 2009, 74, 727–753. [Google Scholar] [CrossRef] [Green Version]
- AnvariFar, H.; Amirkolaie, A.K.; Miandare, H.K.; Ouraji, H.; Jalali, M.A.; Üçüncü, S.İ. Apoptosis in fish: Environmental factors and programmed cell death. Cell Tissue Res. 2017, 368, 425–439. [Google Scholar] [CrossRef]
- Tomalty, K.M.; Meek, M.H.; Stephens, M.R.; Rincón, G.; Fangue, N.A.; May, B.P.; Baerwald, M.R. Transcriptional response to acute thermal exposure in juvenile chinook salmon determined by RNAseq. G3 Genes Genomes Genet. 2015, 5, 1335–1349. [Google Scholar] [CrossRef] [Green Version]
- Jóźwiak, Z.; PaŁecz, D. Influence of heat treatment on osmotic fragility of carp erythrocytes. Int. J. Radiat. Biol. 1988, 54, 299–303. [Google Scholar] [CrossRef]
- Kiron, V.; Takeuchi, T.; Watanabe, T. The osmotic fragility of erythrocytes in rainbow trout under different dietary fatty acid status. Fish. Sci. 1994, 60, 93–95. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Kumar, V.; Kumar, V.; Behera, B.K. Acute phase proteins and their potential role as an indicator for fish health and in diagnosis of fish diseases. Protein Pept. Lett. 2017, 24, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Rebl, A.; Verleih, M.; Köbis, J.M.; Kühn, C.; Wimmers, K.; Köllner, B.; Goldammer, T. Transcriptome profiling of gill tissue in regionally bred and globally farmed rainbow trout strains reveals different strategies for coping with thermal stress. Mar. Biotechnol. 2013, 15, 445–460. [Google Scholar] [CrossRef]
- Rebl, A.; Verleih, M.; Nipkow, M.; Altmann, S.; Bochert, R.; Goldammer, T. Gradual and acute temperature rise induces crossing endocrine, metabolic, and immunological pathways in Maraena whitefish (Coregonus maraena). Front. Genet. 2018, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Casanovas, P.; Walker, S.P.; Johnston, H.; Johnston, C.; Symonds, J.E. Comparative assessment of blood biochemistry and haematology normal ranges between Chinook salmon (Oncorhynchus tshawytscha) from seawater and freshwater farms. Aquaculture 2021, 537, 736464. [Google Scholar] [CrossRef]
- Kammerer, B.D.; Cech, J.J., Jr.; Kültz, D. Rapid changes in plasma cortisol, osmolality, and respiration in response to salinity stress in tilapia (Oreochromis mossambicus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 157, 260–265. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Regish, A.M.; Weinstock, A.; McCormick, S.D. Effects of elevated temperature on osmoregulation and stress responses in Atlantic salmon Salmo salar smolts in fresh water and seawater. J. Fish Biol. 2018, 93, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Chacoff, L.; Arjona, F.J.; Ruiz-Jarabo, I.; García-Lopez, A.; Flik, G.; Mancera, J.M. Water temperature affects osmoregulatory responses in gilthead sea bream (Sparus aurata L.). J. Therm. Biol. 2020, 88, 102526. [Google Scholar] [CrossRef] [PubMed]
- Peres, H.; Santos, S.; Oliva-Teles, A. Selected plasma biochemistry parameters in gilthead seabream (Sparus aurata) juveniles. J. Appl. Ichthyol. 2013, 29, 630–636. [Google Scholar] [CrossRef]
- Racicot, J.G.; Gaudet, M.; Leray, C. Blood and liver enzymes in rainbow trout (Salmo gairdneri Rich.) with emphasis on their diagnostic use: Study of CCl4 toxicity and a case of Aeromonas infection. J. Fish Biol. 1975, 7, 825–835. [Google Scholar] [CrossRef]
- Lowery, M.S.; Somero, G.N. Starvation effects on protein synthesis in red and white muscle of the barred sand bass, Paralabrax Nebulifer. Physiol. Zool. 1990, 63, 630–648. [Google Scholar] [CrossRef]
- Chandra, S. Effect of starvation on serum acid phosphatase levels of freshwater catfish Clarias Batrachus. Experientia 1982, 38, 827–828. [Google Scholar] [CrossRef]
- Ray, C.S.; Singh, B.; Jena, I.; Behera, S.; Ray, S. Low alkaline phosphatase (ALP) in adult population an indicator of zinc (Zn) and magnesium (Mg) deficiency. Curr. Res. Nutr. Food Sci. J. 2017, 5, 347–352. [Google Scholar]
- Amri, A.; Kessabi, K.; Bouraoui, Z.; Sakli, S.; Gharred, T.; Guerbej, H.; Messaoudi, I.; Jebali, J. Effect of melatonin and folic acid supplementation on the growth performance, antioxidant status, and liver histology of the farmed gilthead sea bream (Sparus aurata L.) under standard rearing conditions. Fish Physiol. Biochem. 2020, 46, 2265–2280. [Google Scholar] [CrossRef]
- Barrett, P.V. The effect of diet and fasting on the serum bilirubin concentration in the rat. Gastroenterology 1971, 60, 572–576. [Google Scholar] [CrossRef]
- Sakai, T.; Gotoh, O.; Noguchi, M.; Kawatsu, H. Changes in bilirubin contents and composition in the bile of starved and CCl4 injected carp. Fish Pathol. 1985, 20, 469–473. [Google Scholar] [CrossRef]
- Meyer, B.H.; Scholtz, H.E.; Schall, R.; Muller, F.O.; Hundt, H.K.; Maree, J.S. The effect of fasting on total serum bilirubin concentrations. Br. J. Clin. Pharmacol. 1995, 39, 169–171. [Google Scholar] [CrossRef] [Green Version]
- Rios, F.S.; Oba, E.T.; Fernandes, M.N.; Kalinin, A.L.; Rantin, F.T. Erythrocyte senescence and haematological changes induced by starvation in the neotropical fish traíra, Hoplias malabaricus (Characiformes, Erythrinidae). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 140, 281–287. [Google Scholar] [CrossRef]
- Heming, T.A.; Paleczny, E.J. Compositional changes in skin mucus and blood serum during starvation of trout. Aquaculture 1987, 66, 265–273. [Google Scholar] [CrossRef]
- Mejía-Toiber, J.; Montiel, T.; Massieu, J. D-[β]-hydroxybutyrate prevents glutamate-mediated lipoperoxidation and neuronal damage elicited during glycolysis inhibition in vivo. Neurochem. Res. 2006, 31, 1399–1408. [Google Scholar] [CrossRef]
- Zahl, I.H.; Kiessling, A.; Samuelsen, O.B.; Olsen, R.E. Anesthesia induces stress in Atlantic salmon (Salmo salar), Atlantic cod (Gadus morhua) and Atlantic halibut (Hippoglossus hippoglossus). Fish Physiol. Biochem. 2010, 36, 719–730. [Google Scholar] [CrossRef]
- Zahl, I.H.; Samuelsen, O.; Kiessling, A. Anaesthesia of farmed fish: Implications for welfare. Fish Physiol. Biochem. 2012, 38, 201–218. [Google Scholar] [CrossRef]
- Readman, G.D.; Owen, S.F.; Murrell, J.C.; Knowles, T.G. Do fish perceive anaesthetics as aversive? PLoS ONE 2013, 8, 73773. [Google Scholar] [CrossRef] [Green Version]
- Young, T.; Walker, S.P.; Alfaro, A.C.; Fletcher, L.M.; Murray, J.S.; Lulijwa, R.; Symonds, J. Impact of acute handling stress, anaesthesia, and euthanasia on fish plasma biochemistry: Implications for veterinary screening and metabolomic sampling. Fish Physiol. Biochem. 2019, 45, 1485–1494. [Google Scholar] [CrossRef]
- Madaro, A.; Olsen, R.E.; Kristiansen, T.S.; Ebbesson, L.O.; Nilsen, T.O.; Flik, G.; Gorissen, M. Stress in Atlantic salmon: Response to unpredictable chronic stress. J. Exp. Biol. 2015, 218, 2538–2550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bermejo-Poza, R.; De la Fuente, J.; Pérez, C.; de Chavarri, E.G.; Diaz, M.T.; Torrent, F.; Villarroel, M. Determination of optimal degree days of fasting before slaughter in rainbow trout (Oncorhynchus mykiss). Aquaculture 2017, 473, 272–277. [Google Scholar] [CrossRef] [Green Version]
- Conde-Sieira, M.; Chivite, M.; Míguez, J.M.; Soengas, J.L. Stress effects on the mechanisms regulating appetite in teleost fish. Front. Endocrinol. 2018, 9, 631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkoff, H. Fish as models for understanding the vertebrate endocrine regulation of feeding and weight. Mol. Cell. Endocrinol. 2019, 497, 110437. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, C.; Silva-Marrero, J.I.; Salgado, M.C.; Baanante, I.V.; Metón, I. Role of upstream stimulatory factor 2 in glutamate dehydrogenase gene transcription. J. Mol. Endocrinol. 2018, 60, 247–259. [Google Scholar] [PubMed] [Green Version]
- Liu, L.; Liang, X.-F.; Fang, J.; Li, J. The differentia of nitrogen utilization between fast growth individuals and slow growth individuals in hybrid of Siniperca chuatsi (♀) × Siniperca scherzeri (♂) mandarin fish fed minced prey fish. Aquac. Res. 2016, 48, 4590–4595. [Google Scholar] [CrossRef]
- Kaleeswaran, B.; Ilavenil, S.; Ravikumar, S. Changes in biochemical, histological and specific immune parameters in Catla catla (Ham.) by Cynodon dactylon (L.). J. King Saud Univ. Sci. 2012, 24, 139–152. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.M.; Ali, A.H. Serum proteins and leucocytes differential count in common carp (Cyprinus carpio L.) infested with ectoparasites. Mesop. J. Mar. Sci. 2013, 28, 151–162. [Google Scholar]
- Haghighi, M.; Sharif Rohani, M.; Pourmoghim, H.; Samadi, M.; Tavoli, M.; Eslami, M.; Yusefi, R. Enhancement of immune responses of rainbow trout (Oncorhynchus mykiss) fed a diet supplemented with Aloe vera extract. Iran. J. Fish. Sci. 2017, 17, 884–896. [Google Scholar]
- Kumar, S.; Raman, R.P.; Prasad, K.P.; Srivastava, P.P.; Kumar, S.; Rajendran, K.V. Modulation of innate immune responses and induction of oxidative stress biomarkers in Pangasianodon hypophthalmus following an experimental infection with dactylogyrid monogeneans. Fish Shellfish. Immunol. 2017, 63, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawwab, M.; Hamed, H.S. Effect of bisphenol A toxicity on growth performance, biochemical variables, and oxidative stress biomarkers of Nile tilapia, Oreochromis niloticus (L.). J. Appl. Ichthyol. 2018, 34, 1117–1125. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Begum, B.A. Serum total protein, albumin and A/G ratio in different grades of protein energy malnutrition. Mymensingh Med. J. MMJ 2005, 14, 38–40. [Google Scholar]
- Vosylienė, M.Z. The effect of heavy metals on haematological indices of fish (survey). Acta Zoologica Lituanica 1999, 9, 76–82. [Google Scholar] [CrossRef]
- Witeska, M. Anemia in teleost fishes. Bull. Eur. Assoc. Fish Pathol. 2015, 35, 148–160. [Google Scholar]
- Ciepliński, M.; Kasprzak, M.; Grandtke, M.; Steliga, A.; Kamiński, P.; Jerzak, L. The effect of dipotassium EDTA and lithium heparin on hematologic values of farmed brown trout Salmo trutta (L.) spawners. Aquac. Int. 2019, 27, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Walker, S.P.; Ingram, M.; Bailey, J.; Dodds, K.G.; Fisher, P.J.; Amer, P.R.; Symonds, J.E. In Chinook salmon (Oncorhynchus tshawytscha) feed conversion efficiency: Evaluation and potential for selection. Proc. New Zealand Soc. Anim. Prod. 2012, 72, 227–230. [Google Scholar]
- Perrott, M.R.; Symonds, J.E.; Walker, S.P.; Hely, F.S.; Wybourne, B.; Preece, M.A.; Davie, P.S. Spinal curvatures and onset of vertebral deformities in farmed Chinook salmon, Oncorhynchus tshawytscha (Walbaum, 1792) in New Zealand. J. Appl. Ichthyol. 2018, 34, 501–511. [Google Scholar] [CrossRef]
- Esmaeili, M.; Carter, C.G.; Wilson, R.; Walker, S.P.; Miller, M.R.; Bridle, A.; Symonds, J.E. Proteomic investigation of liver and white muscle in efficient and inefficient Chinook salmon (Oncorhynchus tshawytscha): Fatty acid metabolism and protein turnover drive feed efficiency. Aquaculture 2021, 542, 736855. [Google Scholar] [CrossRef]
- Houston, A.H. Are the classical hematological variables acceptable indicators of fish health? Trans. Am. Fish. Soc. 1997, 126, 879–894. [Google Scholar] [CrossRef]
- Sarkar, M.; Barari, S.K.; Mandal, D.B.; Nandankar, U.A.; Basu, A.; Mohanty, T.K.; Ray, S. The effect of anti-coagulants on the osmotic fragility of erythrocytes in the yak (Poephagus grunniens). Vet. J. 1999, 157, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Lulijwa, R.; Alfaro, A.C.; Merien, F.; Burdass, M.; Young, T.; Meyer, J.; Nguyen, T.V.; Trembath, C. Characterisation of Chinook salmon (Oncorhynchus tshawytscha) blood and validation of flow cytometry cell count and viability assay kit. Fish Shellfish. Immunol. 2019, 88, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Lulijwa, R.; Alfaro, A.C.; Merien, F.; Burdass, M.; Venter, L.; Young, T. In vitro immune response of chinook salmon (Oncorhynchus tshawytscha) peripheral blood mononuclear cells stimulated by bacterial lipopolysaccharide. Fish Shellfish. Immunol. 2019, 94, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Paredes, M.; Gonzalez, K.; Figueroa, J.; Montiel-Eulefi, E. Immunomodulatory effect of prolactin on Atlantic salmon (Salmo salar) macrophage function. Fish. Physiol. Biochem. 2013, 39, 1215–1221. [Google Scholar] [CrossRef]
- Dalmo, R.A.; Seljelid, R. The immunomodulatory effect of LPS, laminaran and sulphated laminaran [β (l,3)-D-glucan] on Atlantic salmon, Salmo salar L., macrophages in vitro. J. Fish. Dis. 1995, 18, 175–185. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lulijwa, R.; Young, T.; Symonds, J.E.; Walker, S.P.; Delorme, N.J.; Alfaro, A.C. Uncoupling Thermotolerance and Growth Performance in Chinook Salmon: Blood Biochemistry and Immune Capacity. Metabolites 2021, 11, 547. https://doi.org/10.3390/metabo11080547
Lulijwa R, Young T, Symonds JE, Walker SP, Delorme NJ, Alfaro AC. Uncoupling Thermotolerance and Growth Performance in Chinook Salmon: Blood Biochemistry and Immune Capacity. Metabolites. 2021; 11(8):547. https://doi.org/10.3390/metabo11080547
Chicago/Turabian StyleLulijwa, Ronald, Tim Young, Jane E. Symonds, Seumas P. Walker, Natalí J. Delorme, and Andrea C. Alfaro. 2021. "Uncoupling Thermotolerance and Growth Performance in Chinook Salmon: Blood Biochemistry and Immune Capacity" Metabolites 11, no. 8: 547. https://doi.org/10.3390/metabo11080547
APA StyleLulijwa, R., Young, T., Symonds, J. E., Walker, S. P., Delorme, N. J., & Alfaro, A. C. (2021). Uncoupling Thermotolerance and Growth Performance in Chinook Salmon: Blood Biochemistry and Immune Capacity. Metabolites, 11(8), 547. https://doi.org/10.3390/metabo11080547