Metabolomics Reveals Process of Allergic Rhinitis Patients with Single- and Double-Species Mite Subcutaneous Immunotherapy
Abstract
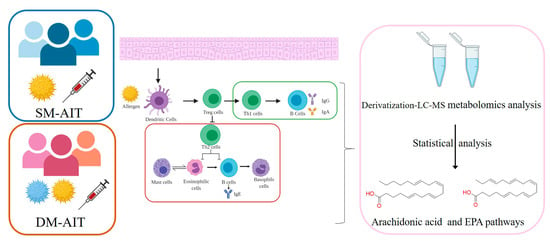
:1. Introduction
2. Results
2.1. Patients
2.2. Clinical Efficacy
2.3. Metabolomics Analysis of Potential Systemic Biomarkers in AR Patients with SM-SCIT or DM-SCIT
2.4. The Change Degree of Metabolites during SM-SCIT and DM-SCIT
2.5. Association between Biomarkers and Clinical Response
3. Discussion
4. Materials and Methods
4.1. Study Design and Population
4.2. Inclusion and Exclusion Criteria
4.3. Clinical Response
4.4. Immunologic Response
4.4.1. Skin Prick Testing
4.4.2. Immunoglobulins
4.5. Sample Preparation
4.6. UHPLC-Q-TOF-MS Analysis
4.7. Data Preprocessing and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rondon, C.; Campo, P.; Eguiluz-Gracia, I.; Plaza, C.; Bogas, G.; Galindo, P.; Mayorga, C.; Torres, M.J. Local allergic rhinitis is an independent rhinitis phenotype: The results of a 10-year follow-up study. Allergy 2018, 73, 470–478. [Google Scholar] [CrossRef]
- Li, J.; Sun, B.; Huang, Y.; Lin, X.; Zhao, D.; Tan, G.; Wu, J.; Zhao, H.; Cao, L.; Zhong, N.; et al. A multicentre study assessing the prevalence of sensitizations in patients with asthma and/or rhinitis in China. Allergy 2009, 64, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, H.; Chen, Y.; Zheng, J.; Wong, G.W.; Zhong, N. House dust mite sensitization is the main risk factor for the increase in prevalence of wheeze in 13- to 14-year-old schoolchildren in Guangzhou city, China. Clin. Exp. Allergy 2013, 43, 1171–1179. [Google Scholar] [PubMed]
- Liu, X.; Zheng, P.; Zheng, S.G.; Zhai, Y.; Zhao, X.; Chen, Y.; Cai, C.; Wu, Z.; Huang, Z.; Zou, X.; et al. Co-sensitization and cross-reactivity of Blomia tropicalis with two Dermatophagoides species in Guangzhou, China. J. Clin. Lab. Anal. 2019, 33, e22981. [Google Scholar] [CrossRef] [Green Version]
- Jutel, M.; Agache, I.; Bonini, S.; Burks, A.W.; Calderon, M.; Canonica, G.W.; Cox, L.; Demoly, P.; Frew, A.J.; O’Hehir, R.; et al. International consensus on allergy immunotherapy. J. Allergy Clin. Immunol. 2015, 136, 556–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yukselen, A.; Kendirli, S.G.; Yilmaz, M.; Altintas, D.U.; Karakoc, G.B.; Yılmaz, M.; Altıntaş, D.U. Effect of one-year subcutaneous and sublingual immunotherapy on clinical and laboratory parameters in children with rhinitis and asthma: A randomized, placebo-controlled, double-blind, double-dummy study. Int. Arch. Allergy Immunol. 2012, 157, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Radulovic, S.; Wilson, D.; Calderon, M.; Durham, S. Systematic reviews of sublingual immunotherapy (SLIT). Allergy 2011, 66, 740–752. [Google Scholar] [CrossRef]
- Nelson, H.; Cartier, S.; Allen-Ramey, F.; Lawton, S.; Calderon, M.A. Network Meta-analysis Shows Commercialized Subcutaneous and Sublingual Grass Products Have Comparable Efficacy. J. Allergy Clin. Immunol. Pract. 2015, 3, 256–266.e3. [Google Scholar] [CrossRef]
- Chelladurai, Y.; Suarez-Cuervo, C.; Erekosima, N.; Kim, J.M.; Ramanathan, M.; Segal, J.B.; Lin, S.Y. Sublingual immunotherapy for the treatment of allergic rhinoconjunctivitis and asthma: A systematic review. JAMA 2013, 309, 1278–1288. [Google Scholar]
- Kim, J.M.; Lin, S.Y.; Suarez-Cuervo, C.; Chelladurai, Y.; Ramanathan, M.; Segal, J.B.; Erekosima, N. Allergen-specific immunotherapy for pediatric asthma and rhinoconjunctivitis: A systematic review. Pediatrics 2013, 131, 1155–1167. [Google Scholar] [CrossRef] [Green Version]
- Di Bona, D.; Plaia, A.; Leto-Barone, M.S.; La Piana, S.; Di Lorenzo, G. Efficacy of subcutaneous and sublingual immunotherapy with grass allergens for seasonal allergic rhinitis: A meta-analysis-based comparison. J. Allergy Clin. Immunol. 2012, 130, 1097–1107.e1092. [Google Scholar] [CrossRef]
- Tabar, A.I.; Arroabarren, E.; Echechipía, S.; García, B.E.; Martin, S.; Alvarez-Puebla, M.J. Three years of specific immunotherapy may be sufficient in house dust mite respiratory allergy. J. Allergy Clin. Immunol. 2011, 127, 57–63.e3. [Google Scholar] [CrossRef]
- Petersen, K.D.; Kronborg, C.; Larsen, J.N.; Dahl, R.; Gyrd-Hansen, D. Patient related outcomes in a real life prospective follow up study: Allergen immunotherapy increase quality of life and reduce sick days. World Allergy Organ. J. 2013, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Calderon, M.; Cardona, V.; Demoly, P.; EAACI 100 Years of Immunotherapy Experts Panel. One hundred years of allergen immunotherapy European Academy of Allergy and Clinical Immunology celebration: Review of unanswered questions. Allergy 2012, 67, 462–476. [Google Scholar] [CrossRef]
- Bousquet, J.; Lockey, R.; Malling, H.J. Allergen immunotherapy: Therapeutic vaccines for allergic diseases. A WHO position paper. J. Allergy Clin. Immunol. 1998, 102, 558–562. [Google Scholar] [CrossRef]
- Mailing, H.-J.; Weeke, B. Position paper: Immunotherapy. Allergy 1993, 48, 9–35. [Google Scholar] [CrossRef]
- Cox, L.; Nelson, H.; Lockey, R.; Calabria, C.; Chacko, T.; Finegold, I.; Nelson, M.; Weber, R.; Bernstein, D.I.; Blessing-Moore, J.; et al. Allergen immunotherapy: A practice parameter third update. J. Allergy Clin. Immunol. 2011, 127, S1–S55. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohe, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Petasis, N.A. Resolvins and protectins in inflammation resolution. Chem. Rev. 2011, 111, 5922–5943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeds, M.C.; Peachman, K.K.; Bowton, D.L.; Sivertson, K.L.; Chilton, F.H. Regulation of arachidonate remodeling enzymes impacts eosinophil survival during allergic asthma. Am. J. Respir. Cell Mol. Biol. 2009, 41, 358–366. [Google Scholar] [CrossRef] [Green Version]
- Reddy, C.N.; Ye, Q.; Patel, P.; Sivendran, S.; Chourey, S.; Wang, R.; Anumolu, J.R.; Grant, G.E.; Powell, W.; Rokach, J. Design and synthesis of affinity chromatography ligands for the purification of 5-hydroxyeicosanoid dehydrogenase. Bioorg. Med. Chem. 2017, 25, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Patel, P.; Reddy, C.N.; Gore, V.; Chourey, S.; Ye, Q.; Ouedraogo, Y.P.; Gravel, S.; Powell, W.S.; Rokach, J. Two Potent OXE-R Antagonists: Assignment of Stereochemistry. ACS Med. Chem. Lett. 2014, 5, 815–819. [Google Scholar] [CrossRef] [Green Version]
- Powell, W.S.; Rokach, J. The eosinophil chemoattractant 5-oxo-ETE and the OXE receptor. Prog Lipid Res 2013, 52, 651–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunnström, Å.; Tryselius, Y.; Feltenmark, S.; Andersson, E.; Leksell, H.; James, A.; Mannervik, B.; Dahlén, B.; Claesson, H.-E. On the biosynthesis of 15-HETE and eoxin C4 by human airway epithelial cells. Prostaglandins Other Lipid. Mediat. 2015, 121, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Mabalirajan, U.; Rehman, R.; Ahmad, T.; Kumar, S.; Leishangthem, G.D.; Singh, S.; Dinda, A.K.; Biswal, S.; Agrawal, A.; Ghosh, B. 12/15-lipoxygenase expressed in non-epithelial cells causes airway epithelial injury in asthma. Sci. Rep. 2013, 3, 1540. [Google Scholar] [CrossRef] [Green Version]
- Bian, X.; Sun, B.; Zheng, P.; Li, N.; Wu, J.-L. Derivatization enhanced separation and sensitivity of long chain-free fatty acids: Application to asthma using targeted and non-targeted liquid chromatography-mass spectrometry approach. Anal. Chim. Acta 2017, 989, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Spertini, F. Metabolomics and allergy: Opening Pandora’s box. J. Allergy Clin. Immunol. 2020, 145, 782–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turi, K.; Romick-Rosendale, L.; Ryckman, K.K.; Hartert, T.V. A review of metabolomics approaches and their application in identifying causal pathways of childhood asthma. J. Allergy Clin. Immunol. 2018, 141, 1191–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.; Li, X.; Zhou, Q.; Quan, C.; Xue, F.; Zheng, J.; Yu, Y. Exploration of candidate biomarkers for human psoriasis based on gas chromatography-mass spectrometry serum metabolomics. Br. J. Dermatol. 2017, 176, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Adamko, D.J.; Nair, P.; Mayers, I.; Tsuyuki, R.T.; Regush, S.; Rowe, B.H. Metabolomic profiling of asthma and chronic obstructive pulmonary disease: A pilot study differentiating diseases. J. Allergy Clin. Immunol. 2015, 136, 571–580 e573. [Google Scholar] [CrossRef]
- Kelly, R.S.; Dahlin, A.; McGeachie, M.J.; Qiu, W.; Sordillo, J.; Wan, E.S.; Wu, A.C.; Lasky-Su, J. Asthma Metabolomics and the Potential for Integrative Omics in Research and the Clinic. Chest 2017, 151, 262–277. [Google Scholar] [CrossRef] [Green Version]
- Reisdorph, N.; Wechsler, M.E. Utilizing metabolomics to distinguish asthma phenotypes: Strategies and clinical implications. Allergy 2013, 68, 959–962. [Google Scholar] [CrossRef]
- Gu, W.Y.; Liu, M.X.; Sun, B.Q.; Guo, M.Q.; Wu, J.L.; Li, N. Profiling of polyunsaturated fatty acids in human serum using off-line and on-line solid phase extraction-nano-liquid chromatography-quadrupole-time-of-flight mass spectrometry. J. Chromatogr. A 2018, 1537, 141–146. [Google Scholar] [CrossRef]
- Szczeklik, W.; Sanak, M.; Mastalerz, L.; Sokołowska, B.M.; Gielicz, A.; Soja, J.; Kumik, J.; Musiał, J.; Szczeklik, A. 12-hydroxy-eicosatetraenoic acid (12-HETE): A biomarker of Churg-Strauss syndrome. Clin. Exp. Allergy 2012, 42, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Jiang, S.; Zhang, H.; Wang, F.; Liu, Y.; She, Y.; Jing, Q.; Gao, K.; Fan, R.; Xie, S.; et al. Prediction of sublingual immunotherapy efficacy in allergic rhinitis by serum metabolomics analysis. Int. Immunopharmacol. 2021, 90, 107211. [Google Scholar] [CrossRef]
- Schneider, C.; Pozzi, A. Cyclooxygenases and lipoxygenases in cancer. Cancer Metastasis Rev. 2011, 30, 277–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, K.S.; Galano, J.-M.; Oger, C.; Durand, T.; Lee, J.C.-Y. Enrichment of alpha-linolenic acid in rodent diet reduced oxidative stress and inflammation during myocardial infarction. Free Radic. Biol. Med. 2021, 162, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.H.; Hsiao, G.; Al-Shabrawey, M. Eicosanoids and Oxidative Stress in Diabetic Retinopathy. Antioxidants 2020, 9, 520. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Fu, X.; Chen, Q.; Patra, J.K.; Wang, D.; Wang, Z.; Gai, Z. Arachidonic Acid Metabolism and Kidney Inflammation. Int. J. Mol. Sci. 2019, 20, 3683. [Google Scholar] [CrossRef] [Green Version]
- Powell, W.S.; Rokach, J. Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid. Biochim. Biophys. Acta 2015, 1851, 340–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mabalirajan, U.; Aich, J.; Leishangthem, G.D.; Sharma, S.K.; Dinda, A.K.; Ghosh, B. Effects of vitamin E on mitochondrial dysfunction and asthma features in an experimental allergic murine model. J. Appl. Physiol. 2009, 107, 1285–1292. [Google Scholar] [CrossRef]
- Ramis, I.; Catafau, J.; Serra, J.; Bulbena, O.; Picado, C.; Gelpí, E. In vivo release of 15-HETE and other arachidonic acid metabolites in nasal secretions during early allergic reactions. Prostaglandins 1991, 42, 411–420. [Google Scholar] [CrossRef]
- Zheng, P.; Bian, X.; Zhai, Y.; Li, C.; Li, N.; Hao, C.; Huang, H.; Luo, W.; Huang, Z.; Liao, C.; et al. Metabolomics reveals a correlation between hydroxyeicosatetraenoic acids and allergic asthma: Evidence from three years’ immunotherapy. Pediat. Allergy Immunol. 2021, 1–9. [Google Scholar] [CrossRef]
- Wang, W.T.; Yang, T.; Song, L.; Guo, M.; Li, C.; Yang, B.; Wang, M.; Kou, N.; Gao, J.; Qu, H.; et al. Combination of Panax notoginseng saponins and aspirin potentiates platelet inhibi-tion with alleviated gastric injury via modulating arachidonic acid metabolism. Biomed. Pharmacother. 2021, 134, 111165. [Google Scholar] [CrossRef]
- Zu, L.; Guo, G.; Zhou, B.; Gao, W. Relationship between metabolites of arachidonic acid and prognosis in patients with acute coronary syndrome. Thromb. Res. 2016, 144, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mazzone, P.J.; Cata, J.P.; Kurz, A.; Bauer, M.; Mascha, E.J.; Sessler, D.I. Serum free fatty acid biomarkers of lung cancer. Chest 2014, 146, 670–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickens, A.; Sordillo, L.M.; Zhang, C.; Fenton, J.I. Obesity is positively associated with arachidonic acid-derived 5- and 11-hydroxyeicosatetraenoic acid (HETE). Metabolism 2017, 70, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Barker-Tejeda, T.C.; Bazire, R.; Obeso, D.; Mera-Berriatua, L.; Rosace, D.; Vazquez-Cortes, S.; Ramos, T.; Rico, M.D.P.; Chivato, T.; Barbas, C.; et al. Exploring novel systemic biomarker approaches in grass-pollen sublingual immunotherapy using omics. Allergy 2021, 76, 1199–1212. [Google Scholar] [CrossRef]
- Esselun, C.; Dilberger, B.; Silaidos, C.V.; Koch, E.; Schebb, N.H.; Eckert, G.P. A Walnut Diet in Combination with Enriched Environment Improves Cognitive Function and Affects Lipid Metabolites in Brain and Liver of Aged NMRI Mice. Neuromol. Med. 2021, 23, 140–160. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Feng, X.; Wang, Z.; Xia, Z. Dietary supplementation with Clostridium butyricum modulates serum lipid metabolism, meat quality, and the amino acid and fatty acid composition of Peking ducks. Poult. Sci. 2018, 97, 3218–3229. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhu, J.-X.; Guo, Y.-M.; Niu, M.; Zhang, L.; Tu, C.; Huang, Y.; Li, P.-Y.; Zhao, X.; Zhang, Z.-T.; et al. Epigallocatechin Gallate During Dietary Restriction—Potential Mechanisms of Enhanced Liver Injury. Front. Pharmacol. 2021, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Juniper, E.F.; Thompson, A.K.; Ferrie, P.J.; Roberts, J.N. Validation of the standardized version of the Rhinoconjunctivitis Quality of Life Questionnaire. J. Allergy Clin. Immunol. 1999, 104, 364–369. [Google Scholar] [CrossRef]






| Characteristics | SM-SCIT | DM-SCIT | p |
|---|---|---|---|
| No. | 35 | 38 | - |
| Sex (Male), No. (%) | 22 (62.9) | 27 (71.1) | 0.456 |
| Age (years), median (IQR) | 11.00 (2.5) | 10.50 (6.3) | 0.881 |
| <18 years, No. (%) | 32 (91.4) | 31 (81.6) | 0.378 |
| AR combined with allergic asthma, No. (%) | 1 (2.9) | 2 (5.3) | 1.000 |
| Atopic family history a, No. (%) | 20 (57.1) | 19(50.0) | 0.541 |
| Score, median (IQR) | |||
| Overall VAS | 24.60 (8.6) | 25.40 (8.3) | 0.782 |
| Sneezing | 5.10 (2.5) | 5.00 (3.8) | 0.760 |
| Runny nose | 5.50 (2.7) | 5.00 (3.0) | 0.650 |
| Blocked nose | 5.10 (2.9) | 5.00 (3.0) | 0.130 |
| Itchy nose | 5.10 (2.4) | 4.80 (2.7) | 0.800 |
| Eye symptoms | 3.80 (2.8) | 3.60 (2.7) | 0.810 |
| Overall RQLQ | 54.00 (27.6) | 51.50 (30.5) | 0.493 |
| Activity limitations | 6.00 (3.7) | 6.00 (3.8) | 0.530 |
| Sleep problems | 5.60 (3.9) | 4.00 (4.5) | 0.680 |
| Non-nose/eye symptoms | 11.90 (7.6) | 12.50 (8.0) | 0.760 |
| Practical problem | 7.90 (3.8) | 7.00 (3.0) | 0.510 |
| Nose symptoms | 10.20 (5.1) | 11.50 (4.3) | 0.210 |
| Eye symptoms | 4.00 (4.0) | 5.40 (4.7) | 0.530 |
| Emotional function | 7.30 (5.9) | 7.20 (5.4) | 0.910 |
| Classification of severity, No. (%) | |||
| Mild intermittent | 12 (34.3) | 13 (34.2) | 0.995 |
| Mild persistent | 8 (22.9) | 9 (23.7) | 0.933 |
| Moderate/severe intermittent | 11 (31.4) | 10 (26.3) | 0.630 |
| Moderate/severe persistent | 4 (11.4) | 6 (15.8) | 0.841 |
| SPT, SI/No.(%) | |||
| Der p, Median (IQR) | 3.00 (1.0) | 3.00 (1.0) | 0.450 |
| Der f, Median (IQR) | 3.00 (1.0) | 3.00 (0.8) | 0.077 |
| Blo t, Median (IQR) | 2.00 (2.5) | 0.00 (2.0) | 0.991 |
| Animal allergens, No. (%) | 8 (22.9) | 12 (31.6) | 0.404 |
| Grass pollens, No. (%) | 1 (2.9) | 5 (13.2) | 0.240 |
| Mold allergens, No. (%) | 0 (0.0) | 3 (7.9) | 0.241 |
| sIgE (IU/mL), median (IQR) | |||
| Der p-sIgE | 15.81 (35.1) | 26.27(58.1) | 0.306 |
| Der f-sIgE | 18.87 (26.8) | 22.52 (36.4) | 0.456 |
| Blo t-sIgE | 0.20 (0.2) | 0.25 (0.2) | 0.716 |
| sIgG4 (U/mL), median (IQR) | |||
| Der p-sIgG4 | 34.17 (7.5) | 35.17 (8.3) | 0.134 |
| Der f-sIgG4 | 164.89 (201.4) | 127.91 (321.3) | 0.216 |
| Blo t-sIgG4 | 41.78 (12.4) | 36.82 (12.5) | 0.298 |
| Compound Name | Formula | Metabolized from | Enzyme | Pathways | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| Linoleic Acid Metabolism | α-Linolenic Acid Metabolism | AA Metabolism | DM | SM | ||||
| 15(S)-HETE | C20H32O3 | AA | 15-LOX,GPX4 | √ | 0.0004 *** | 0.005 ** | ||
| 11(S)-HETE | C20H32O3 | AA | 11-LOX,GPX4 | √ | 0.001 ** | 0.053 | ||
| 12(S)-HETE | C20H32O3 | AA | 12-LOX,GPX4 | √ | 0.313 | 0.422 | ||
| 8(S)-HETE | C20H32O3 | AA | 8-LOX,GPX4 | √ | 0.002 ** | 0.052 | ||
| 5(S)-HETE | C20H32O3 | AA | 5-LOX,GPX4 | √ | 0.001 ** | 0.014 * | ||
| 13(S)-HPODE | C18H32O4 | Linoleic acid | 15-LOX | √ | 0.701 | 0.265 | ||
| 9(S)-HPODE | C18H32O4 | Linoleic acid | 9-LOX | √ | 0.519 | 0.025 * | ||
| 15(S)-HEPE | C20H30O3 | EPA | 15-LOX,GPX4 | √ | 0.617 | 0.154 | ||
| 12(S)-HEPE | C20H30O3 | EPA | 12-LOX,GPX4 | √ | 0.027 * | 0.018 * | ||
| 5(S)-HEPE | C20H30O3 | EPA | 5-LOX,GPX4 | √ | 0.009** | 0.057 | ||
| 13-HODE | C18H32O3 | Linoleic acid | 15-LOX | √ | 0.004 ** | 0.020 * | ||
| AA | C20H32O2 | Linoleic acid | Delta6-desaturase | √ | 0.002 ** | 0.219 | ||
| 13(S)-HOTrE | C18H30O3 | Linoleic acid | 13-LOX | √ | 0.491 | 0.069 | ||
| TXB2 | C20H34O6 | AA | COX | √ | 0.607 | 0.225 | ||
| 12(S)-HHTrE | C17H28O3 | AA | COX | √ | 0.597 | 0.768 | ||
| 11-dehydro TXB2 | C20H32O6 | AA | COX | √ | 0.882 | 0.518 | ||
| EPA | C20H30O2 | α-Linolenic acid | Delta6-desaturase | √ | 0.032 * | 0.207 | ||
| α-Linolenic acid | C18H30O2 | - | √ | 0.0004 *** | 0.302 | |||
| Variables (∆) | ∆ Overall RQLQ | |
|---|---|---|
| r | p | |
| 15(S)-HETE | 0.424 | 0.0002 *** |
| 11(S)-HETE | 0.418 | 0.0002 *** |
| 11-dehydro TXB2 | 0.374 | 0.0011 ** |
| 5(S)-HETE | 0.363 | 0.0016 ** |
| 8(S)-HETE | 0.360 | 0.0017 ** |
| 13-HODE or isomer | 0.353 | 0.0021 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, P.; Yan, G.; Zhang, Y.; Huang, H.; Luo, W.; Xue, M.; Li, N.; Wu, J.-L.; Sun, B. Metabolomics Reveals Process of Allergic Rhinitis Patients with Single- and Double-Species Mite Subcutaneous Immunotherapy. Metabolites 2021, 11, 613. https://doi.org/10.3390/metabo11090613
Zheng P, Yan G, Zhang Y, Huang H, Luo W, Xue M, Li N, Wu J-L, Sun B. Metabolomics Reveals Process of Allergic Rhinitis Patients with Single- and Double-Species Mite Subcutaneous Immunotherapy. Metabolites. 2021; 11(9):613. https://doi.org/10.3390/metabo11090613
Chicago/Turabian StyleZheng, Peiyan, Guanyu Yan, Yida Zhang, Huimin Huang, Wenting Luo, Mingshan Xue, Na Li, Jian-Lin Wu, and Baoqing Sun. 2021. "Metabolomics Reveals Process of Allergic Rhinitis Patients with Single- and Double-Species Mite Subcutaneous Immunotherapy" Metabolites 11, no. 9: 613. https://doi.org/10.3390/metabo11090613
APA StyleZheng, P., Yan, G., Zhang, Y., Huang, H., Luo, W., Xue, M., Li, N., Wu, J. -L., & Sun, B. (2021). Metabolomics Reveals Process of Allergic Rhinitis Patients with Single- and Double-Species Mite Subcutaneous Immunotherapy. Metabolites, 11(9), 613. https://doi.org/10.3390/metabo11090613