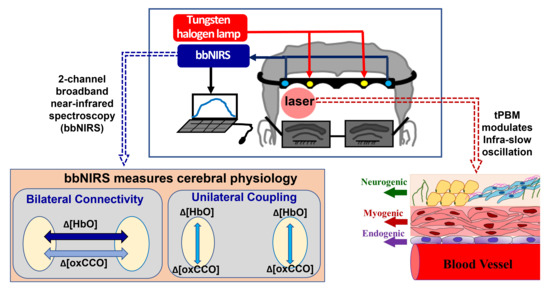
Metabolic Connectivity and Hemodynamic-Metabolic Coherence of Human Prefrontal Cortex at Rest and Post Photobiomodulation Assessed by Dual-Channel Broadband NIRS
Abstract
:1. Introduction
2. Results
2.1. Determination of ISO Coherence among Prefrontal Δ[HbO] and Δ[oxCCO] at Rest
2.1.1. CWT Analysis for ISO Amplitude of Prefrontal Δ[HbO] and Δ[oxCCO] at Rest
2.1.2. WTC Analysis for ISO Coherence among Prefrontal Δ[HbO] and Δ[oxCCO] at Rest
2.2. tPBM-Induced Alterations in ISO Coherence among Prefrontal Δ[HbO] and Δ[oxCCO]
2.2.1. tPBM-Induced Changes in IA of ISO of Prefrontal Δ[HbO] and Δ[oxCCO]
2.2.2. tPBM-Induced Changes in IC of ISO among Prefrontal Δ[HbO] and Δ[oxCCO]
3. Discussion
3.1. Feasibility of bbNIRS in Monitoring Spectral Amplitudes of Human Cerebral ISO at Rest
3.2. Feasibility of bbNIRS to Detect Human ISO Coherence of Prefrontal Cortex at Rest
3.3. bbNIRS as a Promising Non-Invasive Tool for Absolute Value Detections
3.4. tPBM-Enhanced Spectral Amplitude of Ipsilateral Δ[HbO] and Bilateral Δ[oxCCO] ISO
3.5. tPBM-Induced Alteration in Bilateral Functional Connectivity and Unilateral Coherence in ISO of [HbO] and [oxCCO]
3.6. Effects of Sitting on Frequency-Specific ISO
3.7. Limitations and Future Works
4. Materials and Methods
4.1. Participants
4.2. Experiment Protocol and Dual-Channel bbNIRS Setup
4.3. tPBM at 1064 nm Delivered on Right Forehead
4.4. Data Analysis
4.4.1. CWT Analysis for the Human Prefrontal Cortex at Rest
4.4.2. WTC Analysis for the Human Prefrontal Cortex at Rest
4.4.3. Normalization of tPBM-Induced Changes in IA Values
4.4.4. Baseline Subtraction of tPBM-Induced Changes in IC Values
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raichle, M.E.; Gusnard, D.A. Appraising the brain’s energy budget. Proc. Natl. Acad. Sci. USA 2002, 99, 10237–10239. [Google Scholar] [CrossRef] [Green Version]
- Magistretti, P.; Allaman, I. Brain energy metabolism. In Neuroscience in the 21st Century: From Basic to Clinical; Springer: New York, NY, USA, 2013; pp. 1591–1620. [Google Scholar]
- Mateo, C.; Knutsen, P.M.; Tsai, P.S.; Shih, A.Y.; Kleinfeld, D. Entrainment of Arteriole Vasomotor Fluctuations by Neural Activity Is a Basis of Blood-Oxygenation-Level-Dependent “Resting-State” Connectivity. Neuron 2017, 96, 936–948.e3. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.H.; Zhang, N.; Zhang, Y.; Ugurbil, K.; Chen, W. New insights into central roles of cerebral oxygen metabolism in the resting and stimulus-evoked brain. J. Cereb. Blood Flow Metab. 2009, 29, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Brown, L.A.; Key, B.J.; Lovick, T.A. Inhibition of vasomotion in hippocampal cerebral arterioles during increases in neuronal activity. Auton. Neurosci. 2002, 95, 137–140. [Google Scholar] [CrossRef]
- Vermeij, A.; Meel-van den Abeelen, A.S.; Kessels, R.P.; van Beek, A.H.; Claassen, J.A. Very-low-frequency oscillations of cerebral hemodynamics and blood pressure are affected by aging and cognitive load. Neuroimage 2014, 85, 608–615. [Google Scholar] [CrossRef] [PubMed]
- van Helden, D.F.; Imtiaz, M.S. Venous Vasomotion. Adv. Exp. Med. Biol. 2019, 1124, 313–328. [Google Scholar] [CrossRef]
- Klocke, F.J. Epicardial coronary artery vasomotion. J. Am. Coll Cardiol. 2010, 56, 1666–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivadulla, C.; de Labra, C.; Grieve, K.L.; Cudeiro, J. Vasomotion and neurovascular coupling in the visual thalamus in vivo. PLoS ONE 2011, 6, e28746. [Google Scholar] [CrossRef] [PubMed]
- Wiedeman, M.P. Effect of venous flow on frequency of venous vasomotion in the bat wing. Circ. Res. 1957, 5, 641–644. [Google Scholar] [CrossRef] [Green Version]
- Buerk, D.G.; Riva, C.E. Vasomotion and spontaneous low-frequency oscillations in blood flow and nitric oxide in cat optic nerve head. Microvasc. Res. 1998, 55, 103–112. [Google Scholar] [CrossRef]
- Bosch, B.M.; Bringard, A.; Ferretti, G.; Schwartz, S.; Igloi, K. Effect of cerebral vasomotion during physical exercise on associative memory, a near-infrared spectroscopy study. Neurophotonics 2017, 4, 041404. [Google Scholar] [CrossRef]
- Vita, J.A.; Keaney, J.F., Jr. Endothelial function: A barometer for cardiovascular risk? Circulation 2002, 106, 640–642. [Google Scholar] [CrossRef] [Green Version]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef]
- Di Marco, L.Y.; Farkas, E.; Martin, C.; Venneri, A.; Frangi, A.F. Is vasomotion in cerebral arteries impaired in Alzheimer’s disease? J. Alzheimer’s Dis. 2015, 46, 35–53. [Google Scholar] [CrossRef] [Green Version]
- Stefanovska, A.; Bracic, M.; Kvernmo, H.D. Wavelet analysis of oscillations in the peripheral blood circulation measured by laser Doppler technique. IEEE Trans. Biomed. Eng. 1999, 46, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Bracic, M.; Stefanovska, A. Wavelet-based analysis of human blood-flow dynamics. Bull. Math. Biol. 1998, 60, 919–935. [Google Scholar] [CrossRef] [PubMed]
- Bernjak, A.; Clarkson, P.B.; McClintock, P.V.; Stefanovska, A. Low-frequency blood flow oscillations in congestive heart failure and after beta1-blockade treatment. Microvasc. Res. 2008, 76, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Mustari, A.; Nakamura, N.; Kawauchi, S.; Sato, S.; Sato, M.; Nishidate, I. RGB camera-based imaging of cerebral tissue oxygen saturation, hemoglobin concentration, and hemodynamic spontaneous low-frequency oscillations in rat brain following induction of cortical spreading depression. Biomed. Opt. Express 2018, 9, 933–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kvernmo, H.D.; Stefanovska, A.; Kirkeboen, K.A.; Kvernebo, K. Oscillations in the human cutaneous blood perfusion signal modified by endothelium-dependent and endothelium-independent vasodilators. Microvasc. Res. 1999, 57, 298–309. [Google Scholar] [CrossRef]
- Zhang, R.; Zuckerman, J.H.; Iwasaki, K.; Wilson, T.E.; Crandall, C.G.; Levine, B.D. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation 2002, 106, 1814–1820. [Google Scholar] [CrossRef] [Green Version]
- Newman, J.M.; Dwyer, R.M.; St-Pierre, P.; Richards, S.M.; Clark, M.G.; Rattigan, S. Decreased microvascular vasomotion and myogenic response in rat skeletal muscle in association with acute insulin resistance. J. Physiol. 2009, 587, 2579–2588. [Google Scholar] [CrossRef] [PubMed]
- Faraci, F.M.; Heistad, D.D. Regulation of the cerebral circulation: Role of endothelium and potassium channels. Physiol. Rev. 1998, 78, 53–97. [Google Scholar] [CrossRef] [PubMed]
- Girouard, H.; Iadecola, C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J. Appl. Physiol. 2006, 100, 328–335. [Google Scholar] [CrossRef]
- Schmidt, J.A.; Borgstrom, P.; Intaglietta, M. Neurogenic modulation of periodic hemodynamics in rabbit skeletal muscle. J. Appl. Physiol. 1993, 75, 1216–1221. [Google Scholar] [CrossRef]
- Damoiseaux, J.S.; Rombouts, S.A.; Barkhof, F.; Scheltens, P.; Stam, C.J.; Smith, S.M.; Beckmann, C.F. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. USA 2006, 103, 13848–13853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, E.W.; Diehl, R.R.; Timmermann, L.; Baron, R.; Deuschl, G.; Mehdorn, H.M.; Zunker, P. Spontaneous oscillations of arterial blood pressure, cerebral and peripheral blood flow in healthy and comatose subjects. Neurol. Res. 1999, 21, 665–669. [Google Scholar] [CrossRef]
- Mayhew, J.; Zheng, Y.; Hou, Y.; Vuksanovic, B.; Berwick, J.; Askew, S.; Coffey, P. Spectroscopic analysis of changes in remitted illumination: The response to increased neural activity in brain. Neuroimage 1999, 10, 304–326. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Luo, Y.X.; Chen, H.Z.; Liu, D.P. Mitochondria, endothelial cell function, and vascular diseases. Front. Physiol. 2014, 5, 175. [Google Scholar] [CrossRef]
- Ferraresi, C.; de Sousa, M.V.; Huang, Y.Y.; Bagnato, V.S.; Parizotto, N.A.; Hamblin, M.R. Time response of increases in ATP and muscle resistance to fatigue after low-level laser (light) therapy (LLLT) in mice. Lasers Med. Sci. 2015, 30, 1259–1267. [Google Scholar] [CrossRef] [Green Version]
- Ferraresi, C.; Kaippert, B.; Avci, P.; Huang, Y.Y.; de Sousa, M.V.; Bagnato, V.S.; Parizotto, N.A.; Hamblin, M.R. Low-level laser (light) therapy increases mitochondrial membrane potential and ATP synthesis in C2C12 myotubes with a peak response at 3–6 h. Photochem. Photobiol. 2015, 91, 411–416. [Google Scholar] [CrossRef]
- Wang, X.; Tian, F.; Soni, S.S.; Gonzalez-Lima, F.; Liu, H. Interplay between up-regulation of cytochrome-c-oxidase and hemoglobin oxygenation induced by near-infrared laser. Sci. Rep. 2016, 6, 30540. [Google Scholar] [CrossRef] [Green Version]
- Obrig, H.; Neufang, M.; Wenzel, R.; Kohl, M.; Steinbrink, J.; Einhaupl, K.; Villringer, A. Spontaneous low frequency oscillations of cerebral hemodynamics and metabolism in human adults. Neuroimage 2000, 12, 623–639. [Google Scholar] [CrossRef]
- Wang, X.; Tian, F.; Reddy, D.D.; Nalawade, S.S.; Barrett, D.W.; Gonzalez-Lima, F.; Liu, H. Up-regulation of cerebral cytochrome-c-oxidase and hemodynamics by transcranial infrared laser stimulation: A broadband near-infrared spectroscopy study. J. Cereb. Blood Flow Metab. 2017, 37, 3789–3802. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, X.; Liu, H.; Zeng, L. Learning Hemodynamic Effect of Transcranial Infrared Laser Stimulation Using Longitudinal Data Analysis. IEEE J. Biomed. Health Inf. 2020, 24, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, T.; Wang, X.; Wu, A.; Kallioniemi, E.; Husain, M.M.; Liu, H. Transcranial Photobiomodulation (tPBM) with 1064-nm Laser to Improve Cerebral Metabolism of the Human Brain In Vivo. Lasers Surg. Med. 2020, 52, 807–813. [Google Scholar] [CrossRef]
- Hamblin, M.R. Shining light on the head: Photobiomodulation for brain disorders. BBA Clin. 2016, 6, 113–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamblin, M.R.; Huang, Y.Y. (Eds.) Photobiomodulation in the Brain; Acamemic Press: San Diago, CA, USA, 2019. [Google Scholar]
- Barrett, D.W.; Gonzalez-Lima, F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience 2013, 230, 13–23. [Google Scholar] [CrossRef]
- Urquhart, E.L.; Wanniarachchi, H.; Wang, X.; Gonzalez-Lima, F.; Alexandrakis, G.; Liu, H. Transcranial photobiomodulation-induced changes in human brain functional connectivity and network metrics mapped by whole-head functional near-infrared spectroscopy in vivo. Biomed. Opt. Express 2020, 11, 5783–5799. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, M.; Chen, X.; Pohmann, R.; Polimeni, J.R.; Scheffler, K.; Rosen, B.R.; Kleinfeld, D.; Yu, X. Ultra-Slow Single-Vessel BOLD and CBV-Based fMRI Spatiotemporal Dynamics and Their Correlation with Neuronal Intracellular Calcium Signals. Neuron 2018, 97, 925–939.e5. [Google Scholar] [CrossRef] [Green Version]
- Cheng, R.; Shang, Y.; Hayes, D., Jr.; Saha, S.P.; Yu, G. Noninvasive optical evaluation of spontaneous low frequency oscillations in cerebral hemodynamics. Neuroimage 2012, 62, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Tankanag, A.V.; Chemeris, N.K. Adaptive wavelet analysis of oscillations of the cutaneous peripheral blood flow in human. Biofizika 2009, 54, 537–544. [Google Scholar]
- Salvi, P.; Faini, A.; Castiglioni, P.; Brunacci, F.; Montaguti, L.; Severi, F.; Gautier, S.; Pretolani, E.; Benetos, A.; Parati, G. Increase in slow-wave vasomotion by hypoxia and ischemia in lowlanders and highlanders. J. Appl. Physiol. 2018, 125, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Eaton, B.; Paradiso, S.; Mina, M.; Hudetz, A.G.; Bolinger, L. Source of low-frequency fluctuations in functional MRI signal. J. Magn. Reson. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2008, 27, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Elwell, C.; Springett, R.; Hillman, E.; Delpy, D.T. Oscillations in cerebral haemodynamics. In Oxygen Transport to Tissue XXI; Springer: Boston, MA, USA, 1999; pp. 57–65. [Google Scholar]
- Motzkin, J.C.; Newman, J.P.; Kiehl, K.A.; Koenigs, M. Reduced prefrontal connectivity in psychopathy. J. Neurosci. 2011, 31, 17348–17357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Racz, F.S.; Mukli, P.; Nagy, Z.; Eke, A. Increased prefrontal cortex connectivity during cognitive challenge assessed by fNIRS imaging. Biomed. Opt. Express 2017, 8, 3842–3855. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.W.; Lim, S.H.; Kim, B.; Kim, E.; Kim, K.; Kyu Park, S.; Seok Byun, Y.; Sakong, J.; Choi, J.W. Prefrontal functional connectivity analysis of cognitive decline for early diagnosis of mild cognitive impairment: A functional near-infrared spectroscopy study. Biomed. Opt. Express 2020, 11, 1725–1741. [Google Scholar] [CrossRef]
- Jobson, D.D.; Hase, Y.; Clarkson, A.N.; Kalaria, R.N. The role of the medial prefrontal cortex in cognition, ageing and dementia. Brain Commun. 2021, 3, fcab125. [Google Scholar] [CrossRef]
- Sampath, D.; Sathyanesan, M.; Newton, S.S. Cognitive dysfunction in major depression and Alzheimer’s disease is associated with hippocampal–prefrontal cortex dysconnectivity. Neuropsychiatr. Dis. Treat. 2017, 13, 1509. [Google Scholar] [CrossRef] [Green Version]
- Fishburn, F.A.; Norr, M.E.; Medvedev, A.V.; Vaidya, C.J. Sensitivity of fNIRS to cognitive state and load. Front. Hum. Neurosci. 2014, 8, 76. [Google Scholar] [CrossRef]
- Sun, J.; Liu, F.; Wang, H.; Yang, A.; Gao, C.; Li, Z.; Li, X. Connectivity properties in the prefrontal cortex during working memory: A near-infrared spectroscopy study. J. Biomed. Opt. 2019, 24, 051410. [Google Scholar] [CrossRef]
- Baker, J.M.; Bruno, J.L.; Gundran, A.; Hosseini, S.M.H.; Reiss, A.L. Correction: fNIRS measurement of cortical activation and functional connectivity during a visuospatial working memory task. PLoS ONE 2018, 13, e0203233. [Google Scholar] [CrossRef]
- Fan, S.; Blanco-Davis, E.; Zhang, J.; Bury, A.; Warren, J.; Yang, Z.; Yan, X.; Wang, J.; Fairclough, S. The Role of the Prefrontal Cortex and Functional Connectivity during Maritime Operations: An fNIRS study. Brain Behav. 2021, 11, e01910. [Google Scholar] [CrossRef]
- Cordes, D.; Haughton, V.M.; Arfanakis, K.; Carew, J.D.; Turski, P.A.; Moritz, C.H.; Quigley, M.A.; Meyerand, M.E. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. Am. J. Neuroradiol. 2001, 22, 1326–1333. [Google Scholar]
- Sasai, S.; Homae, F.; Watanabe, H.; Sasaki, A.T.; Tanabe, H.C.; Sadato, N.; Taga, G. Frequency-specific network topologies in the resting human brain. Front. Hum. Neurosci. 2014, 8, 1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uludag, K.; Steinbrink, J.; Kohl-Bareis, M.; Wenzel, R.; Villringer, A.; Obrig, H. Cytochrome-c-oxidase redox changes during visual stimulation measured by near-infrared spectroscopy cannot be explained by a mere cross talk artefact. Neuroimage 2004, 22, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Bale, G.; Mitra, S.; Meek, J.; Robertson, N.; Tachtsidis, I. A new broadband near-infrared spectroscopy system for in-vivo measurements of cerebral cytochrome-c-oxidase changes in neonatal brain injury. Biomed. Opt. Express 2014, 5, 3450–3466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, A.; Tachtsidis, I.; Kolyva, C.; Cooper, C.E.; Smith, M.; Elwell, C.E. Use of a hybrid optical spectrometer for the measurement of changes in oxidized cytochrome c oxidase concentration and tissue scattering during functional activation. In Oxygen Transport to Tissue XXXIII; Springer: New York, NY, USA, 2012; pp. 119–124. [Google Scholar]
- Leung, T.S.; Tachtsidis, I.; Elwell, C.E.; Tisdall, M.; Pritchard, C.; Smith, M.; Cooper, C. Investigating cross-talk in cytochrome C oxidase concentration quantification using near infrared spectroscopy in a two-layered model. In Biomedical Optics; Optical Society of America: Washington, DC, USA, 2008; p. BSuE77. [Google Scholar]
- Chen, W.L.; Wagner, J.; Heugel, N.; Sugar, J.; Lee, Y.W.; Conant, L.; Malloy, M.; Heffernan, J.; Quirk, B.; Zinos, A.; et al. Functional Near-Infrared Spectroscopy and Its Clinical Application in the Field of Neuroscience: Advances and Future Directions. Front. Neurosci. 2020, 14, 724. [Google Scholar] [CrossRef]
- Buckley, E.M.; Parthasarathy, A.B.; Grant, P.E.; Yodh, A.G.; Franceschini, M.A. Diffuse correlation spectroscopy for measurement of cerebral blood flow: Future prospects. Neurophotonics 2014, 1, 011009. [Google Scholar] [CrossRef] [Green Version]
- Durduran, T.; Yodh, A.G. Diffuse correlation spectroscopy for non-invasive, micro-vascular cerebral blood flow measurement. Neuroimage 2014, 85, 51–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Yang, Z.; Yuan, T.; Feng, W.; Wang, P. A Systemic Review of Functional Near-Infrared Spectroscopy for Stroke: Current Application and Future Directions. Front. Neurol. 2019, 10, 58. [Google Scholar] [CrossRef]
- Yamada, Y.; Suzuki, H.; Yamashita, Y. Time-domain near-infrared spectroscopy and imaging: A review. Appl. Sci. 2019, 9, 1127. [Google Scholar] [CrossRef] [Green Version]
- Fantini, S.; Sassaroli, A. Frequency-Domain Techniques for Cerebral and Functional Near-Infrared Spectroscopy. Front. Neurosci. 2020, 14, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.Y.; Gupta, A.; Vecchio, D.; de Arce, V.J.; Huang, S.F.; Xuan, W.; Hamblin, M.R. Transcranial low level laser (light) therapy for traumatic brain injury. J. Biophotonics 2012, 5, 827–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thunshelle, C.; Hamblin, M.R. Transcranial Low-Level Laser (Light) Therapy for Brain Injury. Photomed. Laser Surg. 2016, 34, 587–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehpour, F.; Mahmoudi, J.; Kamari, F.; Sadigh-Eteghad, S.; Rasta, S.H.; Hamblin, M.R. Brain Photobiomodulation Therapy: A Narrative Review. Mol. Neurobiol. 2018, 55, 6601–6636. [Google Scholar] [CrossRef]
- Kadenbach, B.; Kuhn-Nentwig, L.; Büge, U. Evolution of a regulatory enzyme: Cytochrome-c oxidase (complex IV). In Current Topics in Bioenergetics; Elsevier: Amsterdam, The Netherlands, 1987; Volume 15, pp. 113–161. [Google Scholar]
- Li, Y.; Park, J.-S.; Deng, J.-H.; Bai, Y. Cytochrome c oxidase subunit IV is essential for assembly and respiratory function of the enzyme complex. J. Bioenerg. Biomembr. 2006, 38, 283–291. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Okamoto, K.; Hayashi, Y.; Sheng, M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 2004, 119, 873–887. [Google Scholar] [CrossRef] [Green Version]
- Zanatta, P.; Toffolo, G.M.; Sartori, E.; Bet, A.; Baldanzi, F.; Agarwal, N.; Golanov, E. The human brain pacemaker: Synchronized infra-slow neurovascular coupling in patients undergoing non-pulsatile cardiopulmonary bypass. Neuroimage 2013, 72, 10–19. [Google Scholar] [CrossRef]
- Phillips, A.A.; Chan, F.H.; Zheng, M.M.; Krassioukov, A.V.; Ainslie, P.N. Neurovascular coupling in humans: Physiology, methodological advances and clinical implications. J. Cereb. Blood Flow Metab. 2016, 36, 647–664. [Google Scholar] [CrossRef] [Green Version]
- Kastrup, J.; Bulow, J.; Lassen, N. Vasomotion in human skin before and after local heating recorded with laser Doppler flowmetry. A method for induction of vasomotion. Int. J. Microcirc. Clin. Exp. 1989, 8, 205–215. [Google Scholar]
- Huneau, C.; Benali, H.; Chabriat, H. Investigating Human Neurovascular Coupling Using Functional Neuroimaging: A Critical Review of Dynamic Models. Front. Neurosci. 2015, 9, 467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dmochowski, G.M.; Shereen, A.D.; Berisha, D.; Dmochowski, J.P. Near-Infrared Light Increases Functional Connectivity with a Non-Thermal Mechanism. Cereb Cortex Commun 2020, 1, tgaa004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meadow, W.; Rudinsky, B.; Bell, A.; Lozon, M.; Randle, C.; Hipps, R. The role of prostaglandins and endothelium-derived relaxation factor in the regulation of cerebral blood flow and cerebral oxygen utilization in the piglet: Operationalizing the concept of an essential circulation. Pediatr. Res. 1994, 35, 649–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, S.; Holder, S.; Thijssen, D.; Hopkins, N. Taking Regular Breaks From Sitting Prevents Reductions in Brain Blood Flow. Front. Young Minds 2019, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Reddy, D.D.; Nalawade, S.S.; Pal, S.; Gonzalez-Lima, F.; Liu, H. Impact of heat on metabolic and hemodynamic changes in transcranial infrared laser stimulation measured by broadband near-infrared spectroscopy. Neurophotonics 2018, 5, 011004. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dmochowski, J.P.; Zeng, L.; Kallioniemi, E.; Husain, M.; Gonzalez-Lima, F.; Liu, H. Transcranial photobiomodulation with 1064-nm laser modulates brain electroencephalogram rhythms. Neurophotonics 2019, 6, 025013. [Google Scholar] [CrossRef]
- Wang, X.; Dmochowski, J.; Husain, M.; Gonzalez-Lima, F.; Liu, H. Proceedings# 18. Transcranial infrared brain stimulation modulates EEG alpha power. Brain Stimul. Basic Transl. Clin. Res. Neuromodulation 2017, 10, e67–e69. [Google Scholar]
- Zhang, Z.; Moore, J. Intrinsic feature extraction in the COI of wavelet power spectra of climatic signals. In Proceedings of the 2011 4th International Congress on Image and Signal Processing, Shanghai, China, 15–17 October 2011; pp. 2354–2356. [Google Scholar]
- Aguiar-Conraria, L.; Soares, M.J. The continuous wavelet transform: Moving beyond uni-and bivariate analysis. J. Econ. Surv. 2014, 28, 344–375. [Google Scholar] [CrossRef]
- Grinsted, A.; Moore, J.C.; Jevrejeva, S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Processes Geophys. 2004, 11, 561–566. [Google Scholar] [CrossRef]
- Torrence, C.; Webster, P.J. Interdecadal Changes in the ENSO—Monsoon System. J. Clim. 1999, 12, 2679–2690. [Google Scholar] [CrossRef] [Green Version]
- Maraun, D.; Kurths, J.; Holschneider, M. Nonstationary Gaussian processes in wavelet domain: Synthesis, estimation, and significance testing. Phys. Rev. E 2007, 75, 016707. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Ma, L.-C.; Shahdadian, S.; Wu, A.; Truong, N.C.D.; Liu, H. Metabolic Connectivity and Hemodynamic-Metabolic Coherence of Human Prefrontal Cortex at Rest and Post Photobiomodulation Assessed by Dual-Channel Broadband NIRS. Metabolites 2022, 12, 42. https://doi.org/10.3390/metabo12010042
Wang X, Ma L-C, Shahdadian S, Wu A, Truong NCD, Liu H. Metabolic Connectivity and Hemodynamic-Metabolic Coherence of Human Prefrontal Cortex at Rest and Post Photobiomodulation Assessed by Dual-Channel Broadband NIRS. Metabolites. 2022; 12(1):42. https://doi.org/10.3390/metabo12010042
Chicago/Turabian StyleWang, Xinlong, Liang-Chieh Ma, Sadra Shahdadian, Anqi Wu, Nghi Cong Dung Truong, and Hanli Liu. 2022. "Metabolic Connectivity and Hemodynamic-Metabolic Coherence of Human Prefrontal Cortex at Rest and Post Photobiomodulation Assessed by Dual-Channel Broadband NIRS" Metabolites 12, no. 1: 42. https://doi.org/10.3390/metabo12010042
APA StyleWang, X., Ma, L. -C., Shahdadian, S., Wu, A., Truong, N. C. D., & Liu, H. (2022). Metabolic Connectivity and Hemodynamic-Metabolic Coherence of Human Prefrontal Cortex at Rest and Post Photobiomodulation Assessed by Dual-Channel Broadband NIRS. Metabolites, 12(1), 42. https://doi.org/10.3390/metabo12010042