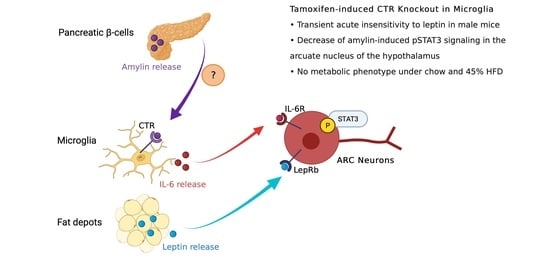
Mouse Microglial Calcitonin Receptor Knockout Impairs Hypothalamic Amylin Neuronal pSTAT3 Signaling but Lacks Major Metabolic Consequences
Abstract
:1. Introduction
2. Results
2.1. Cx3Cr1-CreERT2 Mice Show Specific Cre Activity in Microglia after Tamoxifen Treatment
2.2. CTR Knockout in Microglia Has No Effect on Body Weight and Body Composition
2.3. Amylin and/or Leptin Injections Marginally Affect Food Intake as a Function of Genotype
2.4. CTR Microglial KO Has No Effect on Energy Expenditure but Decreases RER of HFD-Fed Male Mice
2.5. CTR Microglial KO Does Not Alter Glucose Homeostasis
2.6. CTR Microglial KO Impairs Amylin-Induced pSTAT3 in the ARC but Not the Anorectic Response to Leptin
3. Discussion
Effects of Diet, Sex and Tamoxifen
4. Materials and Methods
4.1. Animal Husbandry and Diet
4.2. Transgenic Mice
4.3. Tamoxifen Induction
4.4. Food Intake Measurements after Amylin/Leptin Treatment
4.5. Glucose Tolerance Tests
4.6. Indirect Calorimetry
4.7. Measurement of Body Composition
4.8. Mouse Perfusion
4.9. Immunohistochemistry (IHC)
4.10. Basescope In Situ Hybridization
4.11. Imaging and Quantitative Analysis
4.12. Quantification and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogawa, A.; Harris, V.; McCorkle, S.K.; Unger, R.H.; Luskey, K.L. Amylin secretion from the rat pancreas and its selective loss after streptozotocin treatment. J. Clin. Investig. 1990, 85, 973–976. [Google Scholar] [CrossRef]
- Zakariassen, H.L.; John, L.M.; Lykkesfeldt, J.; Raun, K.; Glendorf, T.; Schaffer, L.; Lundh, S.; Secher, A.; Lutz, T.A.; Le Foll, C. Salmon calcitonin distributes into the arcuate nucleus to a subset of NPY neurons in mice. Neuropharmacology 2020, 167, 107987. [Google Scholar] [CrossRef]
- Lutz, T.A.; Mollet, A.; Rushing, P.A.; Riediger, T.; Scharrer, E. The anorectic effect of a chronic peripheral infusion of amylin is abolished in area postrema/nucleus of the solitary tract (AP/NTS) lesioned rats. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2001, 25, 1005–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potes, C.S.; Turek, V.F.; Cole, R.L.; Vu, C.; Roland, B.L.; Roth, J.D.; Riediger, T.; Lutz, T.A. Noradrenergic neurons of the area postrema mediate amylin’s hypophagic action. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R623–R631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riediger, T.; Schmid, H.A.; Lutz, T.; Simon, E. Amylin potently activates AP neurons possibly via formation of the excitatory second messenger cGMP. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R1833–R1843. [Google Scholar] [CrossRef] [PubMed]
- Lutz, T.A.; Coester, B.; Whiting, L.; Dunn-Meynell, A.A.; Boyle, C.N.; Bouret, S.G.; Levin, B.E.; Le Foll, C. Amylin Selectively Signals Onto POMC Neurons in the Arcuate Nucleus of the Hypothalamus. Diabetes 2018, 67, 805–817. [Google Scholar] [CrossRef] [Green Version]
- Dunn-Meynell, A.A.; Le Foll, C.; Johnson, M.D.; Lutz, T.A.; Hayes, M.R.; Levin, B.E. Endogenous VMH amylin signaling is required for full leptin signaling and protection from diet-induced obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R355–R365. [Google Scholar] [CrossRef]
- Christopoulos, G.; Perry, K.J.; Morfis, M.; Tilakaratne, N.; Gao, Y.; Fraser, N.J.; Main, M.J.; Foord, S.M.; Sexton, P.M. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol. Pharmacol. 1999, 56, 235–242. [Google Scholar] [CrossRef] [Green Version]
- McLatchie, L.M.; Fraser, N.J.; Main, M.J.; Wise, A.; Brown, J.; Thompson, N.; Solari, R.; Lee, M.G.; Foord, S.M. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 1998, 393, 333–339. [Google Scholar] [CrossRef]
- Liberini, C.G.; Boyle, C.N.; Cifani, C.; Venniro, M.; Hope, B.T.; Lutz, T.A. Amylin receptor components and the leptin receptor are co-expressed in single rat area postrema neurons. Eur. J. Neurosci. 2016, 43, 653–661. [Google Scholar] [CrossRef] [Green Version]
- Coester, B.; Koester-Hegmann, C.; Lutz, T.A.; Le Foll, C. Amylin/Calcitonin Receptor-Mediated Signaling in POMC Neurons Influences Energy Balance and Locomotor Activity in Chow-Fed Male Mice. Diabetes 2020, 69, 1110–1125. [Google Scholar] [CrossRef] [PubMed]
- Coester, B.; Pence, S.W.; Arrigoni, S.; Boyle, C.N.; Le Foll, C.; Lutz, T.A. RAMP1 and RAMP3 Differentially Control Amylin’s Effects on Food Intake, Glucose and Energy Balance in Male and Female Mice. Neuroscience 2020, 447, 74–93. [Google Scholar] [CrossRef] [PubMed]
- Arrigoni, S.; Le Foll, C.; Cabak, A.; Lundh, S.; Raun, K.; John, L.M.; Lutz, T.A. A selective role for receptor activity-modifying proteins in subchronic action of the amylin selective receptor agonist NN1213 compared with salmon calcitonin on body weight and food intake in male mice. Eur. J. Neurosci. 2021, 54, 4863–4876. [Google Scholar] [CrossRef]
- Fernandes-Santos, C.; Zhang, Z.; Morgan, D.A.; Guo, D.F.; Russo, A.F.; Rahmouni, K. Amylin acts in the central nervous system to increase sympathetic nerve activity. Endocrinology 2013, 154, 2481–2488. [Google Scholar] [CrossRef] [Green Version]
- Le Foll, C.; Johnson, M.D.; Dunn-Meynell, A.A.; Boyle, C.N.; Lutz, T.A.; Levin, B.E. Amylin-induced central IL-6 production enhances ventromedial hypothalamic leptin signaling. Diabetes 2015, 64, 1621–1631. [Google Scholar] [CrossRef] [Green Version]
- Turek, V.F.; Trevaskis, J.L.; Levin, B.E.; Dunn-Meynell, A.A.; Irani, B.; Gu, G.; Wittmer, C.; Griffin, P.S.; Vu, C.; Parkes, D.G.; et al. Mechanisms of amylin/leptin synergy in rodent models. Endocrinology 2010, 151, 143–152. [Google Scholar] [CrossRef]
- Abegg, K.; Hermann, A.; Boyle, C.N.; Bouret, S.G.; Lutz, T.A.; Riediger, T. Involvement of Amylin and Leptin in the Development of Projections from the Area Postrema to the Nucleus of the Solitary Tract. Front. Endocrinol. 2017, 8, 324. [Google Scholar] [CrossRef] [Green Version]
- Le Foll, C.; Lutz, T.A. Systemic and Central Amylin, Amylin Receptor Signaling, and Their Physiological and Pathophysiological Roles in Metabolism. Compr. Physiol. 2020, 10, 811–837. [Google Scholar] [CrossRef]
- Roth, J.D.; Hughes, H.; Kendall, E.; Baron, A.D.; Anderson, C.M. Antiobesity effects of the beta-cell hormone amylin in diet-induced obese rats: Effects on food intake, body weight, composition, energy expenditure, and gene expression. Endocrinology 2006, 147, 5855–5864. [Google Scholar] [CrossRef] [Green Version]
- Roth, J.D.; Roland, B.L.; Cole, R.L.; Trevaskis, J.L.; Weyer, C.; Koda, J.E.; Anderson, C.M.; Parkes, D.G.; Baron, A.D. Leptin responsiveness restored by amylin agonism in diet-induced obesity: Evidence from nonclinical and clinical studies. Proc. Natl. Acad. Sci. USA 2008, 105, 7257–7262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, B.E.; Lutz, T.A. Amylin and Leptin: Co-Regulators of Energy Homeostasis and Neuronal Development. Trends Endocrinol. Metab. TEM 2017, 28, 153–164. [Google Scholar] [CrossRef]
- Johnson, M.D.; Bouret, S.G.; Dunn-Meynell, A.A.; Boyle, C.N.; Lutz, T.A.; Levin, B.E. Early postnatal amylin treatment enhances hypothalamic leptin signaling and neural development in the selectively bred diet-induced obese rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R1032–R1044. [Google Scholar] [CrossRef] [Green Version]
- Larsen, L.; Le Foll, C.; Dunn-Meynell, A.A.; Levin, B.E. IL-6 ameliorates defective leptin sensitivity in DIO ventromedial hypothalamic nucleus neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R764–R770. [Google Scholar] [CrossRef] [Green Version]
- Madisen, L.; Zwingman, T.A.; Sunkin, S.M.; Oh, S.W.; Zariwala, H.A.; Gu, H.; Ng, L.L.; Palmiter, R.D.; Hawrylycz, M.J.; Jones, A.R.; et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010, 13, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, Y.; Ibata, I.; Ito, D.; Ohsawa, K.; Kohsaka, S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem. Biophys. Res. Commun. 1996, 224, 855–862. [Google Scholar] [CrossRef]
- Ohsawa, K.; Imai, Y.; Sasaki, Y.; Kohsaka, S. Microglia/macrophage-specific protein Iba1 binds to fimbrin and enhances its actin-bundling activity. J. Neurochem. 2004, 88, 844–856. [Google Scholar] [CrossRef]
- Fu, W.; Vukojevic, V.; Patel, A.; Soudy, R.; MacTavish, D.; Westaway, D.; Kaur, K.; Goncharuk, V.; Jhamandas, J. Role of microglial amylin receptors in mediating beta amyloid (Abeta)-induced inflammation. J. Neuroinflamm. 2017, 14, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Febbraio, M.A. gp130 receptor ligands as potential therapeutic targets for obesity. J. Clin. Investig. 2007, 117, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.M.; Chaar, L.J.; Silva, R.C.; Takakura, A.C.; Camara, N.O.; Antunes, V.R.; Moreira, T.S. Minocycline alters expression of inflammatory markers in autonomic brain areas and ventilatory responses induced by acute hypoxia. Exp. Physiol. 2018, 103, 884–895. [Google Scholar] [CrossRef]
- Christopoulos, G.; Paxinos, G.; Huang, X.F.; Beaumont, K.; Toga, A.W.; Sexton, P.M. Comparative distribution of receptors for amylin and the related peptides calcitonin gene related peptide and calcitonin in rat and monkey brain. Can. J. Physiol. Pharmacol. 1995, 73, 1037–1041. [Google Scholar] [CrossRef]
- Ludwig, M.Q.; Cheng, W.; Gordian, D.; Lee, J.; Paulsen, S.J.; Hansen, S.N.; Egerod, K.L.; Barkholt, P.; Rhodes, C.J.; Secher, A.; et al. A genetic map of the mouse dorsal vagal complex and its role in obesity. Nat. Metab. 2021, 3, 530–545. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, B.; Gomez, N.A.; de Avila, J.M.; Zhu, M.J.; Du, M. Even a low dose of tamoxifen profoundly induces adipose tissue browning in female mice. Int. J. Obes. 2020, 44, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Alquier, T.; Poitout, V. Considerations and guidelines for mouse metabolic phenotyping in diabetes research. Diabetologia 2018, 61, 526–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.; Kim, Y.Y.; Nguyen, P.T.-T.; Nam, H.; Suh, J.G. Sex differences in glucose metabolism of streptozotocin-induced diabetes inbred mice (C57BL/6J). Appl. Biol. Chem. 2020, 63, 59. [Google Scholar] [CrossRef]
- Tura, A.; Pacini, G.; Moro, E.; Vrbikova, J.; Bendlova, B.; Kautzky-Willer, A. Sex- and age-related differences of metabolic parameters in impaired glucose metabolism and type 2 diabetes compared to normal glucose tolerance. Diabetes Res. Clin. Pract. 2018, 146, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Yona, S.; Kim, K.W.; Wolf, Y.; Mildner, A.; Varol, D.; Breker, M.; Strauss-Ayali, D.; Viukov, S.; Guilliams, M.; Misharin, A.; et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013, 38, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Keller, J.; Catala-Lehnen, P.; Huebner, A.K.; Jeschke, A.; Heckt, T.; Lueth, A.; Krause, M.; Koehne, T.; Albers, J.; Schulze, J.; et al. Calcitonin controls bone formation by inhibiting the release of sphingosine 1-phosphate from osteoclasts. Nat. Commun. 2014, 5, 5215. [Google Scholar] [CrossRef]
- Coester, B.; Le Foll, C.; Lutz, T.A. Viral depletion of calcitonin receptors in the area postrema: A proof-of-concept study. Physiol. Behav. 2020, 223, 112992. [Google Scholar] [CrossRef]
- Duffy, S.; Lutz, T.A.; Boyle, C.N. Rodent models of leptin receptor deficiency are less sensitive to amylin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R856–R865. [Google Scholar] [CrossRef]
- Weir, J.B. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949, 109, 1–9. [Google Scholar] [CrossRef]
- Luu, S.; Chau, T. Decoding subjective preference from single-trial near-infrared spectroscopy signals. J. Neural Eng. 2009, 6, 016003. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coester, B.; Lutz, T.A.; Le Foll, C. Mouse Microglial Calcitonin Receptor Knockout Impairs Hypothalamic Amylin Neuronal pSTAT3 Signaling but Lacks Major Metabolic Consequences. Metabolites 2022, 12, 51. https://doi.org/10.3390/metabo12010051
Coester B, Lutz TA, Le Foll C. Mouse Microglial Calcitonin Receptor Knockout Impairs Hypothalamic Amylin Neuronal pSTAT3 Signaling but Lacks Major Metabolic Consequences. Metabolites. 2022; 12(1):51. https://doi.org/10.3390/metabo12010051
Chicago/Turabian StyleCoester, Bernd, Thomas A. Lutz, and Christelle Le Foll. 2022. "Mouse Microglial Calcitonin Receptor Knockout Impairs Hypothalamic Amylin Neuronal pSTAT3 Signaling but Lacks Major Metabolic Consequences" Metabolites 12, no. 1: 51. https://doi.org/10.3390/metabo12010051
APA StyleCoester, B., Lutz, T. A., & Le Foll, C. (2022). Mouse Microglial Calcitonin Receptor Knockout Impairs Hypothalamic Amylin Neuronal pSTAT3 Signaling but Lacks Major Metabolic Consequences. Metabolites, 12(1), 51. https://doi.org/10.3390/metabo12010051