Major Nutritional Metabolic Alterations Influencing the Reproductive System of Postpartum Dairy Cows
Abstract
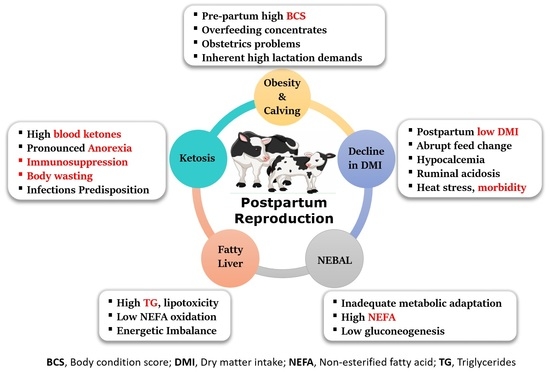
:1. Introduction
2. Nutritional Characteristics, Metabolic Diseases, and Reproduction
3. Fatty Liver
4. The Impact of Ketosis on Reproductive Efficiency of Dairy Cattle
4.1. Ketosis and Ovarian Dynamics
4.2. Ketosis Association with Oocyte Maturation and Implantation
5. The Impact of Hypocalcemia (Milk Fever) on Reproductive Efficiency of Dairy Cattle
6. Ruminal Acidosis and Reproductive Efficiency of Dairy Cattle
7. Effect of High-Protein Diet on Reproductive Performance
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dobson, H.; Smith, R.; Royal, M.; Knight, C.; Sheldon, I. The high-producing dairy cow and its reproductive performance. Reprod. Domest. Anim. 2007, 42 (Suppl. S2), 17–23. [Google Scholar] [CrossRef] [Green Version]
- Lucy, M.C. Reproductive Loss in High-Producing Dairy Cattle: Where Will It End? J. Dairy Sci. 2001, 84, 1277–1293. [Google Scholar] [CrossRef]
- Pryce, J.E.; Royal, M.D.; Garnsworthy, P.C.; Mao, I.L. Fertility in the high-producing dairy cow. Livest. Prod. Sci. 2004, 86, 125–135. [Google Scholar] [CrossRef]
- Lucy, M.C. Symposium review: Selection for fertility in the modern dairy cow—Current status and future direction for genetic selection. Dairy Sci. 2019, 102, 3706–3721. [Google Scholar] [CrossRef] [Green Version]
- Thatcher, W.W.; Bilby, T.R.; Bartolome, J.A.; Silvestre, F.; Staples, C.R.; Santos, J.E.P. Strategies for improving fertility in the modern dairy cow. Theriogenology 2006, 65, 30–44. [Google Scholar] [CrossRef]
- Stevenson, J.S.; Call, E.P. Reproductive Disorders in the Periparturient Dairy Cow. J. Dairy Sci. 1988, 71, 2572–2583. [Google Scholar] [CrossRef]
- Fourichon, C.; Seegers, H.; Malher, X. Effect of disease on reproduction in the dairy cow: A meta-analysis. Theriogenology 2000, 53, 1729–1759. [Google Scholar] [CrossRef]
- Mohtashamipour, F.; Dirandeh, E.; Ansari-pirsaraei, Z.; Colazo, M.G. Postpartum health disorders in lactating dairy cows and its associations with reproductive responses and pregnancy status after first timed-AI. Theriogenology 2020, 141, 98–104. [Google Scholar] [CrossRef]
- Sammad, A.; Umer, S.; Shi, R.; Zhu, H.; Zhao, X.; Wang, Y. Dairy cow reproduction under the influence of heat stress. J. Anim. Physiol. Anim. Nutr. 2020, 104, 978–986. [Google Scholar] [CrossRef]
- Häggman, J.; Christensen, J.M.; Mäntysaari, E.A.; Juga, J. Genetic parameters for endocrine and traditional fertility traits, hyperketonemia and milk yield in dairy cattle. Animal 2019, 13, 248–255. [Google Scholar] [CrossRef] [Green Version]
- Toledo-Alvarado, H.; Cecchinato, A.; Bittante, G. Fertility traits of Holstein, Brown Swiss, Simmental, and Alpine Grey cows are differently affected by herd productivity and milk yield of individual cows. J. Dairy Sci. 2017, 100, 8220–8231. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Z.; Zhao, C.; Bai, Y.; Xia, C.; Xu, C. Effect of negative energy balance on plasma metabolites, minerals, hormones, cytokines and ovarian follicular growth rate in Holstein dairy cows. J. Vet. Res. 2021, 65, 361–368. [Google Scholar] [CrossRef]
- McArt, J.A.A.; Nydam, D.V.; Oetzel, G.R. Epidemiology of subclinical ketosis in early lactation dairy cattle. J. Dairy Sci. 2012, 95, 5056–5066. [Google Scholar] [CrossRef] [Green Version]
- Yoo, H.S. Infectious causes of reproductive disorders in cattle. J. Reprod. Dev. 2010, 56, S53–S60. [Google Scholar] [CrossRef] [Green Version]
- Miqueo, E.; Chiarle, A.; Giuliodori, M.J.; Relling, A.E. Association between prepartum metabolic status and resumption of postpartum ovulation in dairy cows. Domest. Anim. Endocrinol. 2019, 69, 62–67. [Google Scholar] [CrossRef]
- Roth, Z.; Meiden, R.; Braw-Tal, R.; Wolfenson, D. Immediate and delayed effects of heat stress on follicular development and its association with plasma FSH and inhibin concentration in cows. J. Reprod. Fertil. 2000, 120, 83–90. [Google Scholar] [CrossRef]
- LeBlanc, S.J.; Lissemore, K.D.; Kelton, D.F.; Duffield, T.F.; Leslie, K.E. Major advances in disease prevention in dairy cattle. J. Dairy Sci. 2006, 89, 1267–1279. [Google Scholar] [CrossRef] [Green Version]
- LeBlanc, S.J.; Duffield, T.F.; Leslie, K.E.; Bateman, K.G.; Keefe, G.P.; Walton, J.S.; Johnson, W.H. Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J. Dairy Sci. 2002, 85, 2223–2236. [Google Scholar] [CrossRef]
- Vergara, C.F.; Döpfer, D.; Cook, N.B.; Nordlund, K.V.; McArt, J.A.A.; Nydam, D.V.; Oetzel, G.R. Risk factors for postpartum problems in dairy cows: Explanatory and predictive modeling. J. Dairy Sci. 2014, 97, 4127–4140. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Báez, J.; Risco, C.A.; Chebel, R.C.; Gomes, G.C.; Greco, L.F.; Tao, S.; Thompson, I.M.; do Amaral, B.C.; Zenobi, M.G.; Martinez, N.; et al. Association of dry matter intake and energy balance prepartum and postpartum with health disorders postpartum: Part II. Ketosis and clinical mastitis. J. Dairy Sci. 2019, 102, 9151–9164. [Google Scholar] [CrossRef]
- Hayirli, A.; Grummer, R.R.; Nordheim, E.V.; Crump, P.M. Animal and dietary factors affecting feed intake during the prefresh transition period in Holsteins. J. Dairy Sci. 2002, 85, 3430–3443. [Google Scholar] [CrossRef]
- Grummer, R.R.; Mashek, D.G.; Hayirli, A. Dry matter intake and energy balance in the transition period. Vet. Clin. N. Am.-Food Anim. Pract. 2004, 20, 447–470. [Google Scholar] [CrossRef]
- Pérez-Báez, J.; Risco, C.A.; Chebel, R.C.; Gomes, G.C.; Greco, L.F.; Tao, S.; Toledo, I.M.; do Amaral, B.C.; Zenobi, M.G.; Martinez, N.; et al. Investigating the Use of Dry Matter Intake and Energy Balance Prepartum as Predictors of Digestive Disorders Postpartum. Front. Vet. Sci. 2021, 8, 1016. [Google Scholar] [CrossRef]
- Pérez-Báez, J.; Risco, C.A.; Chebel, R.C.; Gomes, G.C.; Greco, L.F.; Tao, S.; Thompson, I.M.; do Amaral, B.C.; Zenobi, M.G.; Martinez, N.; et al. Association of dry matter intake and energy balance prepartum and postpartum with health disorders postpartum: Part I. Calving disorders and metritis. J. Dairy Sci. 2019, 102, 9138–9150. [Google Scholar] [CrossRef]
- Hoedemaker, M.; Prange, D.; Gundelach, Y. Body condition change ante- and postpartum, health and reproductive performance in German Holstein Cows. Reprod. Domest. Anim. 2009, 44, 167–173. [Google Scholar] [CrossRef]
- Chebel, R.C.; Mendonça, L.G.D.; Baruselli, P.S. Association between body condition score change during the dry period and postpartum health and performance. J. Dairy Sci. 2018, 101, 4595–4614. [Google Scholar] [CrossRef]
- Zhang, F.; Li, D.; Wu, Q.; Sun, J.; Guan, W.; Hou, Y.; Zhu, Y.; Wang, J. Prepartum body conditions affect insulin signaling pathways in postpartum adipose tissues in transition dairy cows. J. Anim. Sci. Biotechnol. 2019, 10, 38. [Google Scholar] [CrossRef]
- Roche, J.R.; Meier, S.; Heiser, A.; Mitchell, M.D.; Walker, C.G.; Crookenden, M.A.; Riboni, M.V.; Loor, J.J.; Kay, J.K. Effects of precalving body condition score and prepartum feeding level on production, reproduction, and health parameters in pasture-based transition dairy cows. J. Dairy Sci. 2015, 98, 7164–7182. [Google Scholar] [CrossRef] [Green Version]
- Pires, J.A.A.; Delavaud, C.; Faulconnier, Y.; Pomiès, D.; Chilliard, Y. Effects of body condition score at calving on indicators of fat and protein mobilization of periparturient Holstein-Friesian cows. J. Dairy Sci. 2013, 96, 6423–6439. [Google Scholar] [CrossRef]
- Çolakoğlu, H.E.; Yazlık, M.O.; Pekcan, M.; Kaya, U.; Kaçar, C.; Vural, M.R.; Kurt, S.; Yildirim, M.M.; Bas, A.; Küplülü, Ş. Impact of prepartum body condition score loss on metabolic status during the transition period and subsequent fertility in Brown Swiss dairy cows. J. Vet. Res. 2019, 63, 375. [Google Scholar] [CrossRef] [Green Version]
- Alharthi, A.S.; Coleman, D.N.; Alhidary, I.A.; Abdelrahman, M.M.; Trevisi, E.; Loor, J.J. Maternal body condition during late-pregnancy is associated with in utero development and neonatal growth of Holstein calves. J. Anim. Sci. Biotechnol. 2021, 12, 44. [Google Scholar] [CrossRef]
- Reddy, N.M.; Potteti, H.R.; Vegiraju, S.; Chen, H.J.; Tamatam, C.M.; Reddy, S.P. PI3K-AKT Signaling via Nrf2 Protects against Hyperoxia-Induced Acute Lung Injury, but Promotes Inflammation Post-Injury Independent of Nrf2 in Mice. PLoS ONE 2015, 10, e0129676. [Google Scholar] [CrossRef] [Green Version]
- Bionaz, M.; Chen, S.; Khan, M.J.; Loor, J.J. Functional role of PPARs in ruminants: Potential targets for fine-tuning metabolism during growth and lactation. PPAR Res. 2013, 2013, 684159. [Google Scholar] [CrossRef] [Green Version]
- Shinsyu, A.; Bamba, S.; Kurihara, M.; Matsumoto, H.; Sonoda, A.; Inatomi, O.; Andoh, A.; Takebayashi, K.; Kojima, M.; Iida, H.; et al. Inflammatory cytokines, appetite-regulating hormones, and energy metabolism in patients with gastrointestinal cancer. Oncol. Lett. 2020, 20, 1469–1479. [Google Scholar] [CrossRef]
- Celeska, I.; Janevski, A.; Dzadzovski, I.; Ulchar, I.; Kirovski, D. The dynamics of biochemical parameters in blood of clinically healthy Holstein cows from day 5 before to day 60 after calving. Maced. Vet. Rev. 2015, 38, 189–193. [Google Scholar] [CrossRef] [Green Version]
- Gross, J.J.; Bruckmaier, R.M. Review: Metabolic challenges in lactating dairy cows and their assessment via established and novel indicators in milk. Animal 2019, 13, s75–s81. [Google Scholar] [CrossRef] [Green Version]
- Bach, À. Effects of nutrition and genetics on fertility in dairy cows. Reprod. Fertil. Dev. 2019, 31, 40–54. [Google Scholar] [CrossRef]
- Bauman, D.E.; Bruce Currie, W. Partitioning of Nutrients During Pregnancy and Lactation: A Review of Mechanisms Involving Homeostasis and Homeorhesis. J. Dairy Sci. 1980, 63, 1514–1529. [Google Scholar] [CrossRef]
- Sundrum, A. Metabolic Disorders in the Transition Period Indicate that the Dairy Cows’ Ability to Adapt is Overstressed. Animals 2015, 5, 978–1020. [Google Scholar] [CrossRef]
- Abdelli, A.; Raboisson, D.; Kaidi, R.; Ibrahim, B.; Kalem, A.; Iguer-Ouada, M. Elevated non-esterified fatty acid and β-hydroxybutyrate in transition dairy cows and their association with reproductive performance and disorders: A meta-analysis. Theriogenology 2017, 93, 99–104. [Google Scholar] [CrossRef]
- Cardoso, F.C.; Kalscheur, K.F.; Drackley, J.K. Symposium review: Nutrition strategies for improved health, production, and fertility during the transition period. J. Dairy Sci. 2020, 103, 5684–5693. [Google Scholar] [CrossRef]
- Galligan, D. Economic assessment of animal health performance. Vet. Clin. N. Am.-Food Anim. Pract. 2006, 22, 207–227. [Google Scholar] [CrossRef]
- Ceciliani, F.; Lecchi, C.; Urh, C.; Sauerwein, H. Proteomics and metabolomics characterizing the pathophysiology of adaptive reactions to the metabolic challenges during the transition from late pregnancy to early lactation in dairy cows. J. Proteomics 2018, 178, 92–106. [Google Scholar] [CrossRef]
- Ribeiro, E.S.; Gomes, G.; Greco, L.F.; Cerri, R.L.A.; Vieira-Neto, A.; Monteiro, P.L.J.; Lima, F.S.; Bisinotto, R.S.; Thatcher, W.W.; Santos, J.E.P. Carryover effect of postpartum inflammatory diseases on developmental biology and fertility in lactating dairy cows. J. Dairy Sci. 2016, 99, 2201–2220. [Google Scholar] [CrossRef]
- Carvalho, M.R.; Peñagaricano, F.; Santos, J.E.P.; DeVries, T.J.; McBride, B.W.; Ribeiro, E.S. Long-term effects of postpartum clinical disease on milk production, reproduction, and culling of dairy cows. J. Dairy Sci. 2019, 102, 11701–11717. [Google Scholar] [CrossRef]
- Garzón-Audor, A.; Oliver-Espinosa, O. Incidence and risk factors for ketosis in grazing dairy cattle in the Cundi-Boyacencian Andean plateau, Colombia. Trop. Anim. Health Prod. 2019, 51, 1481–1487. [Google Scholar] [CrossRef]
- Brunner, N.; Groeger, S.; Canelas Raposo, J.; Bruckmaier, R.M.; Gross, J.J. Prevalence of subclinical ketosis and production diseases in dairy cows in Central and South America, Africa, Asia, Australia, New Zealand, and Eastern Europe. Transl. Anim. Sci. 2019, 3, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Sepúlveda-Varas, P.; Weary, D.M.; Noro, M.; Von Keyserlingk, M.A.G. Transition Diseases in Grazing Dairy Cows Are Related to Serum Cholesterol and Other Analytes. PLoS ONE 2015, 10, e0122317. [Google Scholar] [CrossRef] [Green Version]
- Lucy, M.C. Regulation of Ovarian Follicular Growth by Somatotropin and Insulin-Like Growth Factors in Cattle. J. Dairy Sci. 2000, 83, 1635–1647. [Google Scholar] [CrossRef]
- Bauman, D.E.; Vernon, R.G. Effects of Exogenous Bovine Somatotropin on Lactation. Annu. Rev. Nutr. 1993, 13, 437–461. [Google Scholar] [CrossRef]
- Ghanem, M.E.; Tezuka, E.; Sasaki, K.; Takahashi, M.; Yamagishi, N.; Izaike, Y.; Osawa, T. Correlation of blood metabolite concentrations and body condition scores with persistent postpartum uterine bacterial infection in dairy cows. J. Reprod. Dev. 2016, 62, 457–463. [Google Scholar] [CrossRef] [Green Version]
- Beam, S.W.; Butler, W.R. Effects of energy balance on follicular development and first ovulation in postpartum dairy cows. J. Reprod. Fertil. Suppl. 1999, 54, 411–424. [Google Scholar] [CrossRef] [Green Version]
- Rhoads, R.P.; Kim, J.W.; Leury, B.J.; Baumgard, L.H.; Segoale, N.; Frank, S.J.; Bauman, D.E.; Boisclair, Y.R. Insulin Increases the Abundance of the Growth Hormone Receptor in Liver and Adipose Tissue of Periparturient Dairy Cows. J. Nutr. 2004, 134, 1020–1027. [Google Scholar] [CrossRef] [Green Version]
- Jorritsma, R.; Wensing, T.; Kruip, T.A.M.; Vos, P.L.A.M.; Noordhuizen, J.P.T.M. Metabolic changes in early lactation and impaired reproductive performance in dairy cows. Vet. Res. 2003, 34, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Butler, W.R. Nutritional effects on resumption of ovarian cyclicity and conception rate in postpartum dairy cows. BSAP Occas. Publ. 2001, 26, 133–145. [Google Scholar] [CrossRef]
- Castro, N.; Kawashima, C.; van Dorland, H.A.; Morel, I.; Miyamoto, A.; Bruckmaier, R.M. Metabolic and energy status during the dry period is crucial for the resumption of ovarian activity postpartum in dairy cows. J. Dairy Sci. 2012, 95, 5804–5812. [Google Scholar] [CrossRef] [Green Version]
- Villa-Godoy, A.; Hughes, T.L.; Emery, R.S.; Chapin, L.T.; Fogwell, R.L. Association Between Energy Balance and Luteal Function in Lactating Dairy Cows. J. Dairy Sci. 1988, 71, 1063–1072. [Google Scholar] [CrossRef]
- Spicer, L.J.; Tucker, W.B.; Adams, G.D. Insulin-Like Growth Factor-I in Dairy Cows: Relationships Among Energy Balance, Body Condition, Ovarian Activity, and Estrous Behavior. J. Dairy Sci. 1990, 73, 929–937. [Google Scholar] [CrossRef]
- Leroy, J.L.M.R.; Vanholder, T.; Mateusen, B.; Christophe, A.; Opsomer, G.; de Kruif, A.; Genicot, G.; Van Soom, A. Non-esterified fatty acids in follicular fluid of dairy cows and their effect on developmental capacity of bovine oocytes in vitro. Reproduction 2005, 130, 485–495. [Google Scholar] [CrossRef] [Green Version]
- Leroy, J.L.M.R.; Vanholder, T.; Van Knegsel, A.T.M.; Garcia-Ispierto, I.; Bols, P.E.J. Nutrient Prioritization in Dairy Cows Early Postpartum: Mismatch Between Metabolism and Fertility? Reprod. Domest. Anim. 2008, 43, 96–103. [Google Scholar] [CrossRef]
- Seifi, H.A.; Gorji-Dooz, M.; Mohri, M.; Dalir-Naghadeh, B.; Farzaneh, N. Variations of energy-related biochemical metabolites during transition period in dairy cows. Comp. Clin. Path. 2007, 253–258. [Google Scholar] [CrossRef]
- Ospina, P.A.; Nydam, D.V.; Stokol, T.; Overton, T.R. Associations of elevated nonesterified fatty acids and β-hydroxybutyrate concentrations with early lactation reproductive performance and milk production in transition dairy cattle in the northeastern United States. J. Dairy Sci. 2010, 93, 1596–1603. [Google Scholar] [CrossRef] [Green Version]
- Wathes, D.C.; Fenwick, M.; Cheng, Z.; Bourne, N.; Llewellyn, S.; Morris, D.G.; Kenny, D.; Murphy, J.; Fitzpatrick, R. Influence of negative energy balance on cyclicity and fertility in the high producing dairy cow. Theriogenology 2007, 68, S232–S241. [Google Scholar] [CrossRef]
- Velázquez, M.M.L.; Peralta, M.B.; Angeli, E.; Stassi, A.F.; Gareis, N.C.; Durante, L.; Cainelli, S.; Salvetti, N.R.; Rey, F.; Ortega, H.H. Immune status during postpartum, peri-implantation and early pregnancy in cattle: An updated view. Anim. Reprod. Sci. 2019, 206, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zhang, H.; Zhao, Z.; Peng, Z.; Wang, Z.; Liu, G.; Li, X. Non-Esterified Fatty Acids Over-Activate the TLR2/4-NF-Κb Signaling Pathway to Increase Inflammatory Cytokine Synthesis in Neutrophils from Ketotic Cows. Cell. Physiol. Biochem. 2018, 48, 827–837. [Google Scholar] [CrossRef]
- Bradford, B.J.; Yuan, K.; Farney, J.K.; Mamedova, L.K.; Carpenter, A.J. Invited review: Inflammation during the transition to lactation: New adventures with an old flame. J. Dairy Sci. 2015, 98, 6631–6650. [Google Scholar] [CrossRef]
- Wankhade, P.R.; Manimaran, A.; Kumaresan, A.; Jeyakumar, S.; Ramesha, K.P.; Sejian, V.; Rajendran, D.; Varghese, M.R. Metabolic and immunological changes in transition dairy cows: A review. Vet. World 2017, 10, 1367. [Google Scholar] [CrossRef] [Green Version]
- Douglas, G.N.; Overton, T.R.; Bateman, H.G.; Dann, H.M.; Drackley, J.K. Prepartal Plane of Nutrition, Regardless of Dietary Energy Source, Affects Periparturient Metabolism and Dry Matter Intake in Holstein Cows. J. Dairy Sci. 2006, 89, 2141–2157. [Google Scholar] [CrossRef] [Green Version]
- Sammad, A.; Luo, H.; Qiu, W.; Galindez, J.M.; Wang, Y.; Guo, G.; Huang, X.; Wang, Y. Automated monitoring of seasonal and diurnal variation of rumination behaviour: Insights into thermotolerance management of Holstein cows. Biosyst. Eng. 2021. [Google Scholar] [CrossRef]
- Cardoso, F.C.; LeBlanc, S.J.; Murphy, M.R.; Drackley, J.K. Prepartum nutritional strategy affects reproductive performance in dairy cows. J. Dairy Sci. 2013, 96, 5859–5871. [Google Scholar] [CrossRef]
- Janovick, N.A.; Drackley, J.K. Prepartum dietary management of energy intake affects postpartum intake and lactation performance by primiparous and multiparous Holstein cows1. J. Dairy Sci. 2010, 93, 3086–3102. [Google Scholar] [CrossRef]
- Drackley, J.K.; Cicela, T.M.; LaCount, D.W. Responses of primiparous and multiparous holstein cows to additional energy from fat or concentrate during summer. J. Dairy Sci. 2003, 86, 1306–1314. [Google Scholar] [CrossRef] [Green Version]
- Ji, P.; Osorio, J.S.; Drackley, J.K.; Loor, J.J. Overfeeding a moderate energy diet prepartum does not impair bovine subcutaneous adipose tissue insulin signal transduction and induces marked changes in peripartal gene network expression. J. Dairy Sci. 2012, 95, 4333–4351. [Google Scholar] [CrossRef] [Green Version]
- Graugnard, D.E.; Bionaz, M.; Trevisi, E.; Moyes, K.M.; Salak-Johnson, J.L.; Wallace, R.L.; Drackley, J.K.; Bertoni, G.; Loor, J.J. Blood immunometabolic indices and polymorphonuclear neutrophil function in peripartum dairy cows are altered by level of dietary energy prepartum. J. Dairy Sci. 2012, 95, 1749–1758. [Google Scholar] [CrossRef] [Green Version]
- Grummer, R.R. Etiology of Lipid-Related Metabolic Disorders in Periparturient Dairy Cows. J. Dairy Sci. 1993, 76, 3882–3896. [Google Scholar] [CrossRef]
- Ingvartsen, K.L. Feeding- and management-related diseases in the transition cow: Physiological adaptations around calving and strategies to reduce feeding-related diseases. Anim. Feed Sci. Technol. 2006, 126, 175–213. [Google Scholar] [CrossRef]
- Bobe, G.; Young, J.W.; Beitz, D.C. Invited review: Pathology, etiology, prevention, and treatment of fatty liver in dairy cows. J. Dairy Sci. 2004, 87, 3105–3124. [Google Scholar] [CrossRef] [Green Version]
- Drackley, J.K. ADSA foundation scholar award: Biology of dairy cows during the transition period: The final frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, L.; Yang, W.; Wang, Z. Exploration of serum sensitive biomarkers of fatty liver in dairy cows. Sci. Rep. 2018, 8, 13574. [Google Scholar] [CrossRef]
- Jorritsma, R.; Jorritsma, H.; Schukken, Y.H.; Wentink, G.H. Relationships between fatty liver and fertility and some periparturient diseases in commercial Dutch dairy herds. Theriogenology 2000, 54, 1065–1074. [Google Scholar] [CrossRef]
- Trevisi, E.; Amadori, M.; Bakudila, A.M.; Bertoni, G. Metabolic changes in dairy cows induced by oral, low-dose interferon-alpha treatment. J. Anim. Sci. 2009, 87, 3020–3029. [Google Scholar] [CrossRef]
- Loor, J.J.; Everts, R.E.; Bionaz, M.; Dann, H.M.; Morin, D.E.; Oliveira, R.; Rodriguez-Zas, S.L.; Drackley, J.K.; Lewin, H.A. Nutrition-induced ketosis alters metabolic and signaling gene networks in liver of periparturient dairy cows. Physiol. Genom. 2007, 32, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Overton, T.R.; Waldron, M.R. Nutritional management of transition dairy cows: Strategies to optimize metabolic health. J. Dairy Sci. 2004, 87, E105–E119. [Google Scholar] [CrossRef] [Green Version]
- Bionaz, M.; Vargas-Bello-Pérez, E.; Busato, S. Advances in fatty acids nutrition in dairy cows: From gut to cells and effects on performance. J. Anim. Sci. Biotechnol. 2020, 111, 110. [Google Scholar] [CrossRef]
- Thomas, M.G.; Bao, B.; Williams, G.L. Dietary Fats Varying in Their Fatty Acid Composition Differentially Influence Follicular Growth in Cows Fed Isoenergetic Diets. J. Anim. Sci. 1997, 75, 2512–2519. [Google Scholar] [CrossRef]
- Staples, C.R.; Burke, J.M.; Thatcher, W.W. Influence of Supplemental Fats on Reproductive Tissues and Performance of Lactating Cows. J. Dairy Sci. 1998, 81, 856–871. [Google Scholar] [CrossRef]
- Petit, H.V.; Palin, M.F.; Doepel, L. Hepatic Lipid Metabolism in Transition Dairy Cows Fed Flaxseed. J. Dairy Sci. 2007, 90, 4780–4792. [Google Scholar] [CrossRef]
- Zapata, R.C.; Salehi, R.; Ambrose, D.J.; Chelikani, P.K. Effects of prepartum fat supplementation on plasma concentrations of glucagon-like peptide-1, peptide YY, adropin, insulin, and leptin in periparturient dairy cows. J. Dairy Sci. 2015, 98, 6876–6885. [Google Scholar] [CrossRef]
- Karcagi, R.G.; Gaál, T.; Wágner, L.; Husvéth, F. Effect of various dietary fat supplementations on liver lipid and glycogen of high-yielding dairy cows in the peripartal period. Acta Vet. Hung. 2008, 56, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Karimian, M.; Khorvash, M.; Forouzmand, M.A.; Alikhani, M.; Rahmani, H.R.; Ghaffari, M.H.; Petit, H.V. Effect of prepartal and postpartal dietary fat level on performance and plasma concentration of metabolites in transition dairy cows. J. Dairy Sci. 2015, 98, 330–337. [Google Scholar] [CrossRef]
- Santos, J.E.P.; Bilby, T.R.; Thatcher, W.W.; Staples, C.R.; Silvestre, F.T. Long Chain Fatty Acids of Diet as Factors Influencing Reproduction in Cattle. Reprod. Domest. Anim. 2008, 43, 23–30. [Google Scholar] [CrossRef]
- Milojevic, V.; Sinz, S.; Kreuzer, M.; Chiumia, D.; Marquardt, S.; Giller, K. Partitioning of fatty acids into tissues and fluids from reproductive organs of ewes as affected by dietary phenolic extracts. Theriogenology 2020, 144, 174–184. [Google Scholar] [CrossRef]
- Silvestre, F.T.; Carvalho, T.S.M.; Francisco, N.; Santos, J.E.P.; Staples, C.R.; Jenkins, T.C.; Thatcher, W. Effects of differential supplementation of fatty acids during the peripartum and breeding periods of Holstein cows: I. Uterine and metabolic responses, reproduction, and lactation. J. Dairy Sci. 2011, 94, 189–204. [Google Scholar] [CrossRef] [Green Version]
- Zachut, M.; Arieli, A.; Moallem, U. Incorporation of dietary n-3 fatty acids into ovarian compartments in dairy cows and the effects on hormonal and behavioral patterns around estrus. Reproduction 2011, 141, 833–840. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, E.S. Symposium review: Lipids as regulators of conceptus development: Implications for metabolic regulation of reproduction in dairy cattle1. J. Dairy Sci. 2018, 101, 3630–3641. [Google Scholar] [CrossRef] [Green Version]
- Busato, S.; Bionaz, M. The interplay between non-esterified fatty acids and bovine peroxisome proliferator-activated receptors: Results of an in vitro hybrid approach. J. Anim. Sci. Biotechnol. 2020, 11, 91. [Google Scholar] [CrossRef]
- Esposito, G.; Irons, P.C.; Webb, E.C.; Chapwanya, A. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows. Anim. Reprod. Sci. 2014, 144, 60–71. [Google Scholar] [CrossRef] [Green Version]
- Nazeer, M.; Kumar, S.; Jaiswal, M.; Mishra, A.; Upmanyu, G.; Kumar, P.; Kumar, S.A. Prevalence and Clinical Manifestations of Ketosis in Cows in and Around Bikaner. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1554–1560. [Google Scholar] [CrossRef]
- Galster, A.D.; Clutter, W.E.; Cryer, P.E.; Collins, J.A.; Bier, D.M. Epinephrine plasma thresholds for lipolytic effects in man: Measurements of fatty acid transport with [l-13C]palmitic acid. J. Clin. Investig. 1981, 67, 1729–1738. [Google Scholar] [CrossRef]
- Melendez, P.; Marin, M.P.; Robles, J.; Rios, C.; Duchens, M.; Archbald, L. Relationship between serum nonesterified fatty acids at calving and the incidence of periparturient diseases in Holstein dairy cows. Theriogenology 2009, 72, 826–833. [Google Scholar] [CrossRef]
- Duffield, T. Subclinical ketosis in lactating dairy cattle. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 231–253. [Google Scholar] [CrossRef]
- Oetzel, G.R. Monitoring and testing dairy herds for metabolic disease. Vet. Clin. N. Am.-Food Anim. Pract. 2004, 20, 651–674. [Google Scholar] [CrossRef]
- Staufenbiel, R.; Arndt, G.; Schröder, U.; Gelfert, C.C. Body condition and metabolic stability as the basis for high milk yield and undisturbed fertility in dairy cows—A contribution for deduction of reference values. Dtsch. Tierarztl. Wochenschr. 2004, 111, 214–220. [Google Scholar]
- Mostert, P.F.; Bokkers, E.A.M.; Van Middelaar, C.E.; Hogeveen, H.; De Boer, I.J.M. Estimating the economic impact of subclinical ketosis in dairy cattle using a dynamic stochastic simulation model. Animal 2018, 12, 145–154. [Google Scholar] [CrossRef]
- Diskin, M.G.; Mackey, D.R.; Roche, J.F.; Sreenan, J.M. Effects of nutrition and metabolic status on circulating hormones and ovarian follicle development in cattle. Anim. Reprod. Sci. 2003, 78, 345–370. [Google Scholar] [CrossRef]
- Pushpakumara, P.G.A.; Gardner, N.H.; Reynolds, C.K.; Beever, D.E.; Wathes, D.C. Relationships between transition period diet, metabolic parameters and fertility in lactating dairy cows. Theriogenology 2003, 60, 1165–1185. [Google Scholar] [CrossRef]
- Chapinal, N.; LeBlanc, S.J.; Carson, M.E.; Leslie, K.E.; Godden, S.; Capel, M.; Santos, J.E.P.; Overton, M.W.; Duffield, T.F. Herd-level association of serum metabolites in the transition period with disease, milk production, and early lactation reproductive performance. J. Dairy Sci. 2012, 95, 5676–5682. [Google Scholar] [CrossRef]
- Shin, E.K.; Jeong, J.K.; Choi, I.S.; Kang, H.G.; Hur, T.Y.; Jung, Y.H.; Kim, I.H. Relationships among ketosis, serum metabolites, body condition, and reproductive outcomes in dairy cows. Theriogenology 2015, 84, 252–260. [Google Scholar] [CrossRef]
- Lüttgenau, J.; Purschke, S.; Tsousis, G.; Bruckmaier, R.M.; Bollwein, H. Body condition loss and increased serum levels of nonesterified fatty acids enhance progesterone levels at estrus and reduce estrous activity and insemination rates in postpartum dairy cows. Theriogenology 2016, 85, 656–663. [Google Scholar] [CrossRef]
- Rutherford, A.J.; Oikonomou, G.; Smith, R.F. The effect of subclinical ketosis on activity at estrus and reproductive performance in dairy cattle. J. Dairy Sci. 2016, 99, 4808–4815. [Google Scholar] [CrossRef] [Green Version]
- Aardema, H.; van Tol, H.T.A.; Vos, P.L.A.M. An overview on how cumulus cells interact with the oocyte in a condition with elevated NEFA levels in dairy cows. Anim. Reprod. Sci. 2019, 207, 131–137. [Google Scholar] [CrossRef]
- Walsh, S.W.; Williams, E.J.; Evans, A.C.O. A review of the causes of poor fertility in high milk producing dairy cows. Anim. Reprod. Sci. 2011, 123, 127–138. [Google Scholar] [CrossRef]
- Piechotta, M.; Mysegades, W.; Ligges, U.; Lilienthal, J.; Hoeflich, A.; Miyamoto, A.; Bollwein, H. Antepartal insulin-like growth factor 1 and insulin-like growth factor binding protein 2 concentrations are indicative of ketosis in dairy cows. J. Dairy Sci. 2015, 98, 3100–3109. [Google Scholar] [CrossRef] [Green Version]
- Sarentonglaga, B.; Ogata, K.; Taguchi, Y.; Kato, Y.; Nagao, Y. The developmental potential of oocytes is impaired in cattle with liver abnormalities. J. Reprod. Dev. 2013, 59, 168–173. [Google Scholar] [CrossRef] [Green Version]
- Valckx, S.D.M.; Arias-Alvarez, M.; De Pauw, I.; Fievez, V.; Vlaeminck, B.; Fransen, E.; Bols, P.E.J.; Leroy, J.L.M.R. Fatty acid composition of the follicular fluid of normal weight, overweight and obese women undergoing assisted reproductive treatment: A descriptive cross-sectional study. Reprod. Biol. Endocrinol. 2014, 12, 13. [Google Scholar] [CrossRef]
- Aardema, H.; Gadella, B.M.; van de Lest, C.H.A.; Brouwers, J.F.H.M.; Stout, T.A.E.; Roelen, B.A.J.; Vos, P.L.A.M. Free fatty acid levels in fluid of dominant follicles at the preferred insemination time in dairy cows are not affected by early postpartum fatty acid stress. J. Dairy Sci. 2015, 98, 2322–2336. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Liu, S.; Lin, R.; Wang, J.; Peng, T.; Zhang, Q.; Cheng, H. Plasma and amniotic fluid PPARγ is involved in the lipid metabolism of maternal–fetal interface cells. J. Matern. Neonatal Med. 2018, 31, 2656–2664. [Google Scholar] [CrossRef]
- Furukawa, E.; Chen, Z.; Ueshiba, H.; Wu, Y.; Chiba, H.; Yanagawa, Y.; Katagiri, S.; Nagano, M.; Hui, S.P. Postpartum cows showed high oocyte triacylglycerols concurrently with high plasma free fatty acids. Theriogenology 2021, 176, 174–182. [Google Scholar] [CrossRef]
- Campanile, G.; Baruselli, P.S.; Limone, A.; D’Occhio, M.J. Local action of cytokines and immune cells in communication between the conceptus and uterus during the critical period of early embryo development, attachment and implantation – Implications for embryo survival in cattle: A review. Theriogenology 2021, 167, 1–12. [Google Scholar] [CrossRef]
- van Mourik, M.S.M.; Macklon, N.S.; Heijnen, C.J. Embryonic implantation: Cytokines, adhesion molecules, and immune cells in establishing an implantation environment. J. Leukoc. Biol. 2009, 85, 4–19. [Google Scholar] [CrossRef] [Green Version]
- Lacasse, P.; Vanacker, N.; Ollier, S.; Ster, C. Innovative dairy cow management to improve resistance to metabolic and infectious diseases during the transition period. Res. Vet. Sci. 2018, 116, 40–46. [Google Scholar] [CrossRef]
- Aleri, J.W.; Hine, B.C.; Pyman, M.F.; Mansell, P.D.; Wales, W.J.; Mallard, B.; Fisher, A.D. Periparturient immunosuppression and strategies to improve dairy cow health during the periparturient period. Res. Vet. Sci. 2016, 108, 8–17. [Google Scholar] [CrossRef]
- Drackley, J.K.; Cardoso, F.C. Prepartum and postpartum nutritional management to optimize fertility in high-yielding dairy cows in confined TMR systems. Animal 2014, 8, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Garnsworthy, P.C.; Sinclair, K.D.; Webb, R. Integration of physiological mechanisms that influence fertility in dairy cows. Animal 2008, 2, 1144–1152. [Google Scholar] [CrossRef] [Green Version]
- Goff, J.P. The monitoring, prevention, and treatment of milk fever and subclinical hypocalcemia in dairy cows. Vet. J. 2008, 176, 50–57. [Google Scholar] [CrossRef]
- Zhang, F.; Nan, X.; Wang, H.; Guo, Y.; Xiong, B. Research on the applications of calcium propionate in dairy cows: A review. Animals 2020, 10, 1336. [Google Scholar] [CrossRef]
- Hernández-Castellano, L.E.; Hernandez, L.L.; Bruckmaier, R.M. Review: Endocrine pathways to regulate calcium homeostasis around parturition and the prevention of hypocalcemia in periparturient dairy cows. Animal 2020, 14, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Özçelik, R.; Bruckmaier, R.M.; Hernández-Castellano, L.E. Prepartum daylight exposure increases serum calcium concentrations in dairy cows at the onset of lactation. J. Anim. Sci. 2017, 95, 4440–4447. [Google Scholar] [CrossRef] [Green Version]
- Wysolmerski, J.J. Osteocytic osteolysis: Time for a second look? Bonekey Rep. 2012, 1, 229. [Google Scholar] [CrossRef] [Green Version]
- Megahed, A.A.; Hiew, M.W.H.; El Badawy, S.A.; Constable, P.D. Plasma calcium concentrations are decreased at least 9 hours before parturition in multiparous Holstein-Friesian cattle in a herd fed an acidogenic diet during late gestation. J. Dairy Sci. 2018, 101, 1365–1378. [Google Scholar] [CrossRef] [Green Version]
- Umaña Sedó, S.; Rosa, D.; Mattioli, G.; Luzbel de la Sota, R.; Giuliodori, M.J. Associations of subclinical hypocalcemia with fertility in a herd of grazing dairy cows. J. Dairy Sci. 2018, 101, 10469–10477. [Google Scholar] [CrossRef] [Green Version]
- Saborío-Montero, A.; Vargas-Leitón, B.; Romero-Zúñiga, J.J.; Sánchez, J.M. Risk factors associated with milk fever occurrence in grazing dairy cattle. J. Dairy Sci. 2017, 100, 9715–9722. [Google Scholar] [CrossRef] [Green Version]
- Chiwome, B.; Kandiwa, E.; Mushonga, B.; Sajeni, S.; Habarugira, G. A study of the incidence of milk fever in Jersey and Holstein cows at a dairy farm in Beatrice, Zimbabwe. J. S. Afr. Vet. Assoc. 2017, 88, 6. [Google Scholar] [CrossRef] [Green Version]
- DeGaris, P.J.; Lean, I.J. Milk fever in dairy cows: A review of pathophysiology and control principles. Vet. J. 2008, 176, 58–69. [Google Scholar] [CrossRef]
- Venjakob, P.L.; Borchardt, S.; Heuwieser, W. Hypocalcemia—Cow-level prevalence and preventive strategies in German dairy herds. J. Dairy Sci. 2017, 100, 9258–9266. [Google Scholar] [CrossRef] [Green Version]
- Lean, I.J.; DeGaris, P.J.; McNeil, D.M.; Block, E. Hypocalcemia in dairy cows: Meta-analysis and dietary cation anion difference theory revisited. J. Dairy Sci. 2006, 89, 669–684. [Google Scholar] [CrossRef] [Green Version]
- Caixeta, L.S.; Ospina, P.A.; Capel, M.B.; Nydam, D.V. Association between subclinical hypocalcemia in the first 3 days of lactation and reproductive performance of dairy cows. Theriogenology 2017, 94, 1–7. [Google Scholar] [CrossRef]
- Correa, M.T.; Erb, H.; Scarlett, J. Path Analysis for Seven Postpartum Disorders of Holstein Cows. J. Dairy Sci. 1993, 76, 1305–1312. [Google Scholar] [CrossRef]
- Markusfeld, O. Periparturient Traits in Seven High Dairy Herds. Incidence Rates, Association with Parity, and Interrelationships Among Traits. J. Dairy Sci. 1987, 70, 158–166. [Google Scholar] [CrossRef]
- Jeong, J.K.; Kang, H.G.; Kim, I.H. Associations between serum calcium concentration and postpartum health and reproductive performance in dairy cows. Anim. Reprod. Sci. 2018, 196, 184–192. [Google Scholar] [CrossRef]
- Thys-Jacobs, S.; Donovan, D.; Papadopoulos, A.; Sarrel, P.; Bilezikian, J.P. Vitamin D and calcium dysregulation in the polycystic ovarian syndrome. Steroids 1999, 64, 430–435. [Google Scholar] [CrossRef]
- Reinhardt, T.A.; Lippolis, J.D.; McCluskey, B.J.; Goff, J.P.; Horst, R.L. Prevalence of subclinical hypocalcemia in dairy herds. Vet. J. 2011, 188, 122–124. [Google Scholar] [CrossRef]
- Neves, R.C.; Leno, B.M.; Stokol, T.; Overton, T.R.; McArt, J.A.A. Risk factors associated with postpartum subclinical hypocalcemia in dairy cows. J. Dairy Sci. 2017, 100, 3796–3804. [Google Scholar] [CrossRef] [Green Version]
- Chapinal, N.; Carson, M.; Duffield, T.F.; Capel, M.; Godden, S.; Overton, M.; Santos, J.E.P.; LeBlanc, S.J. The association of serum metabolites with clinical disease during the transition period. J. Dairy Sci. 2011, 94, 4897–4903. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, E.M.; Arís, A.; Bach, A. Associations between subclinical hypocalcemia and postparturient diseases in dairy cows. J. Dairy Sci. 2017, 100, 7427–7434. [Google Scholar] [CrossRef]
- Oetzel, G.R. Parturient paresis and hypocalcemia in ruminant livestock. Vet. Clin. N. Am. Food Anim. Pract. 1988, 4, 351–364. [Google Scholar] [CrossRef]
- Leno, B.M.; Ryan, C.M.; Stokol, T.; Kirk, D.; Zanzalari, K.P.; Chapman, J.D.; Overton, T.R. Effects of prepartum dietary cation-anion difference on aspects of peripartum mineral and energy metabolism and performance of multiparous Holstein cows. J. Dairy Sci. 2017, 100, 4604–4622. [Google Scholar] [CrossRef]
- Weich, W.; Block, E.; Litherland, N.B. Extended negative dietary cation-anion difference feeding does not negatively affect postpartum performance of multiparous dairy cows. J. Dairy Sci. 2013, 96, 5780–5792. [Google Scholar] [CrossRef] [Green Version]
- Lopera, C.; Zimpel, R.; Vieira-Neto, A.; Lopes, F.R.; Ortiz, W.; Poindexter, M.; Faria, B.N.; Gambarini, M.L.; Block, E.; Nelson, C.D.; et al. Effects of level of dietary cation-anion difference and duration of prepartum feeding on performance and metabolism of dairy cows. J. Dairy Sci. 2018, 101, 7907–7929. [Google Scholar] [CrossRef]
- Ryan, K.T.; Guadagnin, A.R.; Glosson, K.M.; Bascom, S.S.; Rowson, A.D.; Steelman, A.J.; Cardoso, F.C. Increased dietary calcium inclusion in fully acidified prepartum diets improved postpartum uterine health and fertility when fed to Holstein cows. Theriogenology 2020, 142, 338–347. [Google Scholar] [CrossRef]
- Thilsing-Hansen, T.; Jørgensen, R.J.; Enemark, J.M.D.; Larsen, T. The effect of zeolite a supplementation in the dry period on periparturient calcium, phosphorus, and magnesium homeostasis. J. Dairy Sci. 2002, 85, 1855–1862. [Google Scholar] [CrossRef] [Green Version]
- Kronqvist, C.; Emanuelson, U.; Spörndly, R.; Holtenius, K. Effects of prepartum dietary calcium level on calcium and magnesium metabolism in periparturient dairy cows. J. Dairy Sci. 2011, 94, 1365–1373. [Google Scholar] [CrossRef]
- Kronqvist, C.; Emanuelson, U.; Tråvén, M.; Spãrndly, R.; Holtenius, K. Relationship between incidence of milk fever and feeding of minerals during the last 3 weeks of gestation. Animal 2012, 6, 1316–1321. [Google Scholar] [CrossRef] [Green Version]
- Wächter, S.; Cohrs, I.; Golbeck, L.; Wilkens, M.R.; Grünberg, W. Effects of restricted dietary phosphorus supply to dry cows on periparturient calcium status. J. Dairy Sci. 2022, 105, 748–760. [Google Scholar] [CrossRef]
- Martín-Tereso, J.; Martens, H.; Deiner, C.; Van Laar, H.; Den Hartog, L.A.; Verstegen, M.W.A. Pre-calving feeding of rumen-protected rice bran to multiparous dairy cows improves recovery of calcaemia after calving. J. Dairy Res. 2016, 83, 281–288. [Google Scholar] [CrossRef]
- Khachlouf, K.; Hamed, H.; Gdoura, R.; Gargouri, A. Effects of dietary Zeolite supplementation on milk yield and composition and blood minerals status in lactating dairy cows. J. Appl. Anim. Res. 2019, 47, 54–62. [Google Scholar] [CrossRef]
- Laporta, J.; Moore, S.A.E.; Peters, M.W.; Peters, T.L.; Hernandez, L.L. Short communication: Circulating serotonin (5-HT) concentrations on day 1 of lactation as a potential predictor of transition-related disorders. J. Dairy Sci. 2013, 96, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Slater, C.J.; Endres, E.L.; Weaver, S.R.; Cheng, A.A.; Lauber, M.R.; Endres, S.F.; Olstad, E.; DeBruin, A.; Crump, P.M.; Block, E.; et al. Interaction of 5-hydroxy-L-tryptophan and negative dietary cation-anion difference on calcium homeostasis in multiparous peripartum dairy cows. J. Dairy Sci. 2018, 101, 5486–5501. [Google Scholar] [CrossRef]
- Sato, S. Pathophysiological evaluation of subacute ruminal acidosis (SARA) by continuous ruminal pH monitoring. Anim. Sci. J. 2016, 87, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Steele, M.A.; AlZahal, O.; Hook, S.E.; Croom, J.; McBride, B.W. Ruminal acidosis and the rapid onset of ruminal parakeratosis in a mature dairy cow: A case report. Acta Vet. Scand. 2009, 51, 39. [Google Scholar] [CrossRef] [Green Version]
- Kleen, J.L.; Upgang, L.; Rehage, J. Prevalence and consequences of subacute ruminal acidosis in German dairy herds. Acta Vet. Scand. 2013, 55, 48. [Google Scholar] [CrossRef] [Green Version]
- McCann, J.C.; Luan, S.; Cardoso, F.C.; Derakhshani, H.; Khafipour, E.; Loor, J.J. Induction of subacute ruminal acidosis affects the ruminal microbiome and epithelium. Front. Microbiol. 2016, 7, 701. [Google Scholar] [CrossRef] [Green Version]
- Zebeli, Q.; Ghareeb, K.; Humer, E.; Metzler-Zebeli, B.U.; Besenfelder, U. Nutrition, rumen health and inflammation in the transition period and their role on overall health and fertility in dairy cows. Res. Vet. Sci. 2015, 103, 126–136. [Google Scholar] [CrossRef]
- Plaizier, J.C.; Krause, D.O.; Gozho, G.N.; McBride, B.W. Subacute ruminal acidosis in dairy cows: The physiological causes, incidence and consequences. Vet. J. 2008, 176, 21–31. [Google Scholar] [CrossRef]
- Heidari, M.; Kafi, M.; Mirzaei, A.; Asaadi, A.; Mokhtari, A. Effects of follicular fluid of preovulatory follicles of repeat breeder dairy cows with subclinical endometritis on oocyte developmental competence. Anim. Reprod. Sci. 2019, 205, 62–69. [Google Scholar] [CrossRef]
- Lüttgenau, J.; Herzog, K.; Strüve, K.; Latter, S.; Boos, A.; Bruckmaier, R.M.; Bollwein, H.; Kowalewski, M.P. LPS-mediated effects and spatio-temporal expression of TLR2 and TLR4 in the bovine corpus luteum. Reproduction 2016, 151, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Haziak, K.; Herman, A.P.; Tomaszewska-Zaremba, D. Effects of central injection of anti-LPS antibody and blockade of TLR4 on GnRH/LH secretion during immunological stress in anestrous ewes. Mediators Inflamm. 2014, 2014, 867170. [Google Scholar] [CrossRef]
- Haziak, K.; Herman, A.P.; Wojtulewicz, K.; Pawlina, B.; Paczesna, K.; Bochenek, J.; Tomaszewska-Zaremba, D. Effect of CD14/TLR4 antagonist on GnRH/LH secretion in ewe during central inflammation induced by intracerebroventricular administration of LPS. J. Anim. Sci. Biotechnol. 2018, 9, 52. [Google Scholar] [CrossRef]
- Kadzere, C.; Murphy, M.; Silanikove, N.; Maltz, E. Heat stress in lactating dairy cows: A review. Livest. Prod. Sci. 2002, 77, 59–91. [Google Scholar] [CrossRef]
- Jenkins, T.C. Lipid Metabolism in the Rumen. J. Dairy Sci. 1993, 76, 3851–3863. [Google Scholar] [CrossRef]
- Doreau, M.; Ferlay, A. Digestion and utilisation of fatty acids by ruminants. Anim. Feed Sci. Technol. 1994, 45, 379–396. [Google Scholar] [CrossRef]
- Bauman, D.E.; Griinari, J.M. Nutritional regulation of milk fat synthesis. Annu. Rev. Nutr. 2003, 23, 203–227. [Google Scholar] [CrossRef] [Green Version]
- Jouany, J.P. Optimizing rumen functions in the close-up transition period and early lactation to drive dry matter intake and energy balance in cows. Anim. Reprod. Sci. 2006, 96, 250–264. [Google Scholar] [CrossRef]
- Kamada, H. Effects of selenium-rich yeast supplementation on the plasma progesterone levels of postpartum dairy cows. Asian-Australas. J. Anim. Sci. 2017, 30, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Wildman, C.D.; West, J.W.; Bernard, J.K. Effect of dietary cation-anion difference and dietary crude protein on performance of lactating dairy cows during hot weather. J. Dairy Sci. 2007, 90, 1842–1850. [Google Scholar] [CrossRef]
- Erdman, R.A. Dietary Buffering Requirements of the Lactating Dairy Cow: A Review. J. Dairy Sci. 1988, 71, 3246–3266. [Google Scholar] [CrossRef]
- Wang, F.; Shi, H.; Wang, S.; Wang, Y.; Cao, Z.; Li, S. Amino Acid Metabolism in Dairy Cows and their Regulation in Milk Synthesis. Curr. Drug Metab. 2019, 20, 36–45. [Google Scholar] [CrossRef]
- Butler, W.R. Nutritional interactions with reproductive performance in dairy cattle. Anim. Reprod. Sci. 2000, 60–61, 449–457. [Google Scholar] [CrossRef]
- Tamminga, S. The effect of the supply of rumen degradable protein and metabolisable protein on negative energy balance and fertility in dairy cows. Anim. Reprod. Sci. 2006, 96, 227–239. [Google Scholar] [CrossRef]
- Campanile, G.; Di Palo, R.; Infascelli, F.; Gasparrini, B.; Neglia, G.; Zicarelli, F.; D’Occhio, M.J. Influence of rumen protein degradability on productive and reproductive performance in buffalo cows. Reprod. Nutr. Dev. 2003, 43, 557–566. [Google Scholar] [CrossRef] [Green Version]
- Raboisson, D.; Albaaj, A.; Nonne, G.; Foucras, G. High urea and pregnancy or conception in dairy cows: A meta-analysis to define the appropriate urea threshold. J. Dairy Sci. 2017, 100, 7581–7587. [Google Scholar] [CrossRef] [Green Version]
- Kananub, S.; Pechkerd, P.; VanLeeuwen, J.; Stryhn, H.; Arunvipas, P. Evaluation of influence of milk urea nitrogen on reproductive performance in smallholder dairy farms. Aust. Vet. J. 2020, 98, 375–379. [Google Scholar] [CrossRef]
- Mohanty, P.; Ghanim, H.; Hamouda, W.; Aljada, A.; Garg, R.; Dandona, P. Both lipid and protein intakes stimulate increased generation of reactive oxygen species by polymorphonuclear leukocytes and mononuclear cells. Am. J. Clin. Nutr. 2002, 75, 767–772. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Gao, S.; Quan, S.; Zhang, Y.; Bu, D.; Wang, J. Blood amino acids profile responding to heat stress in dairy cows. Asian-Australas. J. Anim. Sci. 2018, 31, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Baumgard, L.H.; Rhoads, R.P. Effects of Heat Stress on Postabsorptive Metabolism and Energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef] [Green Version]
- Sammad, A.; Wang, Y.J.; Umer, S.; Lirong, H.; Khan, I.; Khan, A.; Ahmad, B.; Wang, Y. Nutritional physiology and biochemistry of dairy cattle under the influence of heat stress: Consequences and opportunities. Animals 2020, 10, 793. [Google Scholar] [CrossRef]
- Gao, H. Amino acids in reproductive nutrition and health. Adv. Exp. Med. Biol. 2020, 1265, 111–131. [Google Scholar]
- Elango, R.; Ball, R.O. Protein and Amino Acid Requirements during Pregnancy. Adv. Nutr. 2016, 7, 839S–844S. [Google Scholar] [CrossRef] [Green Version]
- Ennis, M.; Lim, K.; Ball, R.; Pencharz, P.; Courtney-Martin, G.; Elango, R. Dietary Phenylalanine and Tyrosine Requirements in Healthy Human Pregnancy. Curr. Dev. Nutr. 2020, 4. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Spain, J.N.; Spiers, D.E. Supplementation of Nicotinic Acid for Lactating Holstein Cows Under Heat Stress Conditions. J. Dairy Sci. 1997, 80, 1200–1206. [Google Scholar] [CrossRef]
- Hansen, P.J. Hidden factors affecting fertility. Adv. Dairy Technol. Proc. West. Can. Dairy Semin. 2007, 19, 339–349. [Google Scholar]
- Kaufman, J.D.; Pohler, K.G.; Mulliniks, J.T.; Ríus, A.G. Lowering rumen-degradable and rumen-undegradable protein improved amino acid metabolism and energy utilization in lactating dairy cows exposed to heat stress. J. Dairy Sci. 2018, 101, 386–395. [Google Scholar] [CrossRef] [Green Version]
- Havekes, C.D.; Duffield, T.F.; Carpenter, A.J.; DeVries, T.J. Effects of wheat straw chop length in high-straw dry cow diets on intake, health, and performance of dairy cows across the transition period. J. Dairy Sci. 2020, 103, 254–271. [Google Scholar] [CrossRef]
- Acosta, D.A.V.; Rivelli, M.I.; Skenandore, C.; Zhou, Z.; Keisler, D.H.; Luchini, D.; Corrêa, M.N.; Cardoso, F.C. Effects of rumen-protected methionine and choline supplementation on steroidogenic potential of the first postpartum dominant follicle and expression of immune mediators in Holstein cows. Theriogenology 2017, 96, 1–9. [Google Scholar] [CrossRef]
- Skenandore, C.S.; Velasco Acosta, D.A.; Zhou, Z.; Rivelli, M.I.; Corrêa, M.N.; Luchini, D.N.; Cardoso, F.C. Effects of rumen-protected methionine and choline supplementation on vaginal discharge and uterine cytology of Holstein cows. Int. J. Vet. Sci. Med. 2017, 5, 1–7. [Google Scholar] [CrossRef]
- Stella, S.L.; Velasco-Acosta, D.A.; Skenandore, C.; Zhou, Z.; Steelman, A.; Luchini, D.; Cardoso, F.C. Improved uterine immune mediators in Holstein cows supplemented with rumen-protected methionine and discovery of neutrophil extracellular traps (NET). Theriogenology 2018, 114, 116–125. [Google Scholar] [CrossRef]
- Acosta, D.A.V.; Denicol, A.C.; Tribulo, P.; Rivelli, M.I.; Skenandore, C.; Zhou, Z.; Luchini, D.; Corrêa, M.N.; Hansen, P.J.; Cardoso, F.C. Effects of rumen-protected methionine and choline supplementation on the preimplantation embryo in Holstein cows. Theriogenology 2016, 85, 1669–1679. [Google Scholar] [CrossRef]
- Palmquist, D.L.; Jenkins, T.C. Fat in Lactation Rations: Review. J. Dairy Sci. 1980, 63, 1–14. [Google Scholar] [CrossRef]
- Bicalho, M.L.S.; Lima, F.S.; Ganda, E.K.; Foditsch, C.; Meira, E.B.S.; Machado, V.S.; Teixeira, A.G.V.; Oikonomou, G.; Gilbert, R.O.; Bicalho, R.C. Effect of trace mineral supplementation on selected minerals, energy metabolites, oxidative stress, and immune parameters and its association with uterine diseases in dairy cattle. J. Dairy Sci. 2014, 97, 4281–4295. [Google Scholar] [CrossRef] [Green Version]
- Mammi, L.M.E.; Guadagnini, M.; Mechor, G.; Cainzos, J.M.; Fusaro, I.; Palmonari, A.; Formigoni, A. The Use of Monensin for Ketosis Prevention in Dairy Cows during the Transition Period: A Systematic Review. Animals 2021, 11, 1988. [Google Scholar] [CrossRef]
- Silva, P.R.B.; Machado, K.S.; Da Silva, D.N.L.; Moraes, J.G.N.; Keisler, D.H.; Chebel, R.C. Effects of recombinant bovine somatotropin during the periparturient period on innate and adaptive immune responses, systemic inflammation, and metabolism of dairy cows. J. Dairy Sci. 2015, 98, 4449–4464. [Google Scholar] [CrossRef] [Green Version]
- Wheelock, J.B.; Rhoads, R.P.; VanBaale, M.J.; Sanders, S.R.; Baumgard, L.H. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 2010, 93, 644–655. [Google Scholar] [CrossRef]
- Hansen, P.J.; Aréchiga, C.F. Strategies for managing reproduction in the heat-stressed dairy cow. J. Anim. Sci. 1999, 77 (Suppl. 2), 36–50. [Google Scholar] [CrossRef] [Green Version]
- Cartmill, J.A.; El-Zarkouny, S.Z.; Hensley, B.A.; Rozell, T.G.; Smith, J.F.; Stevenson, J.S. An Alternative AI Breeding Protocol for Dairy Cows Exposed to Elevated Ambient Temperatures before or after Calving or Both. J. Dairy Sci. 2001, 84, 799–806. [Google Scholar] [CrossRef]
- Stewart, B.M.; Block, J.; Morelli, P.; Navarette, A.E.; Amstalden, M.; Bonilla, L.; Hansen, P.J.; Bilby, T.R. Efficacy of embryo transfer in lactating dairy cows during summer using fresh or vitrified embryos produced in vitro with sex-sorted semen. J. Dairy Sci. 2011, 94, 3437–3445. [Google Scholar] [CrossRef] [Green Version]
- López-Gatius, F.; Santolaria, P.; Martino, A.; Delétang, F.; De Rensis, F. The effects of GnRH treatment at the time of AI and 12 days later on reproductive performance of high producing dairy cows during the warm season in northeastern Spain. Theriogenology 2006, 65, 820–830. [Google Scholar] [CrossRef]
- Friedman, E.; Voet, H.; Reznikov, D.; Wolfenson, D.; Roth, Z. Hormonal treatment before and after artificial insemination differentially improves fertility in subpopulations of dairy cows during the summer and autumn. J. Dairy Sci. 2014, 97, 7465–7475. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sammad, A.; Khan, M.Z.; Abbas, Z.; Hu, L.; Ullah, Q.; Wang, Y.; Zhu, H.; Wang, Y. Major Nutritional Metabolic Alterations Influencing the Reproductive System of Postpartum Dairy Cows. Metabolites 2022, 12, 60. https://doi.org/10.3390/metabo12010060
Sammad A, Khan MZ, Abbas Z, Hu L, Ullah Q, Wang Y, Zhu H, Wang Y. Major Nutritional Metabolic Alterations Influencing the Reproductive System of Postpartum Dairy Cows. Metabolites. 2022; 12(1):60. https://doi.org/10.3390/metabo12010060
Chicago/Turabian StyleSammad, Abdul, Muhammad Zahoor Khan, Zaheer Abbas, Lirong Hu, Qudrat Ullah, Yajing Wang, Huabin Zhu, and Yachun Wang. 2022. "Major Nutritional Metabolic Alterations Influencing the Reproductive System of Postpartum Dairy Cows" Metabolites 12, no. 1: 60. https://doi.org/10.3390/metabo12010060
APA StyleSammad, A., Khan, M. Z., Abbas, Z., Hu, L., Ullah, Q., Wang, Y., Zhu, H., & Wang, Y. (2022). Major Nutritional Metabolic Alterations Influencing the Reproductive System of Postpartum Dairy Cows. Metabolites, 12(1), 60. https://doi.org/10.3390/metabo12010060