LC-HRMS-Based Non-Targeted Metabolomics for the Assessment of Honey Adulteration with Sugar Syrups: A Preliminary Study
Abstract
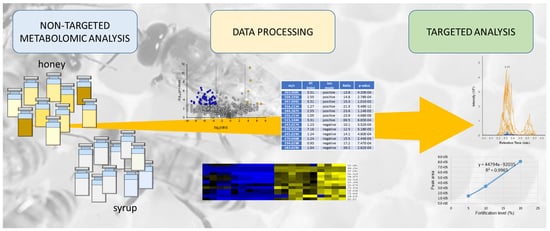
:1. Introduction
2. Materials and Methods
2.1. Reagents, Chemicals, Honey and Syrup Samples
2.2. Sample Preparation
2.3. Liquid Chromatography and High-Resolution Tandem Mass Spectrometry (LC-HRMS)
2.4. Data Processing and Statistical Analysis
2.5. Targeted Analysis of Potential Markers of Honey Adulteration
3. Results
3.1. Non-Targeted Metabolomics Fingerprint
3.2. Targeted Analysis of Potential Markers
3.3. Quantification of the Most Promising Potential Marker
3.4. Analysis of Honey Obtained from Sugar Beet Syrup Overfeeding and Commercial Honey
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crane, E. Honey: A Comprehensive Survey; Heinemann: London, UK, 1975; 608p. [Google Scholar]
- Moore, J.C.; Spink, J.; Lipp, M. Development and application of a database of food ingredient fraud and economically motivated adulteration from 1980 to 2010. J. Food Sci. 2012, 77, R118–R126. [Google Scholar] [CrossRef] [PubMed]
- EU. 2021 Annual Report. Alert and Cooperation Network. 2022. 28p. Available online: https://food.ec.europa.eu/safety/agri-food-fraud/eu-food-fraud-network_en (accessed on 26 July 2022).
- García, N.; Schwarzinger, S. Honey fraud. In Food Fraud: A Global Threat with Public Health and Economic Consequences; Hellberg, R., Everstine, K., Sklare, S., Eds.; Elsevier: London, UK, 2021; pp. 309–334. [Google Scholar] [CrossRef]
- Wakgari, M.; Yigezu, G. Honeybee keeping constraints and future prospects. Cogent Food Agric. 2021, 7, 1872192. [Google Scholar] [CrossRef]
- Grassl, J.; Holt, S.; Cremen, N.; Peso, M.; Hahne, D.; Baer, B. Synergistic effects of pathogen and pesticide exposure on honey bee (Apis mellifera) survival and immunity. J. Invertebr. Pathol. 2018, 159, 78–86. [Google Scholar] [CrossRef]
- EU. Honey. Detailed Information on Honey Production in the European Union. 2022. Available online: https://agriculture.ec.europa.eu/farming/animal-products/honey_en (accessed on 8 August 2022).
- Aries, E.; Burton, J.; Carrasco, L.; De Rudder, O.; Maquet, A. Scientific support to the Implementation of a Coordinated Control Plan with a View to Establishing the Prevalence of Fraudulent Practices in the Marketing of Honey—Results of Honey Authenticity Testing by Liquid Chromatography-Isotope Ratio Mass Spectrometry. JRC Tech Rep 2016 (JRC104749). Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/oc_control-progs_honey_jrc-tech-report_2016.pdf (accessed on 6 July 2022).
- EP. European Parliament. Draft Report on the Food Crisis, Fraud in the Food Chain and the Control Thereof (2013/2091(INI)). 2013. Available online: http://www.europarl.europa.eu/sides/getDoc.do?pubRef=-//EP//NONSGML+COMPARL+PE-519.759+02+DOC+PDF+V0//EN&language=EN (accessed on 10 July 2022).
- EP. European Parliament. European Parliament Resolution of 1 March 2018 on Prospects and Challenges for the EU Apiculture Sector (2017/2115(INI)). 2018. Available online: http://www.europarl.europa.eu/sides/getDoc.do?pubRef=-//EP//TEXT+TA+P8-TA-2018-0057+0+DOC+XML+V0//EN (accessed on 10 July 2022).
- EU. National Apiculture Programmes. 2022. Available online: https://agriculture.ec.europa.eu/farming/animal-products/honey/national-apiculture-programmes_en (accessed on 8 August 2022).
- Wu, L.; Du, B.; Vander Heyden, Y.; Chen, L.; Zhao, L.; Wang, M.; Xue, X. Recent advancements in detecting sugar-based adulterants in honey—A challenge. TrAC Trends Anal. Chem. 2017, 86, 25–38. [Google Scholar] [CrossRef]
- Martinello, M.; Mutinelli, F. Antioxidant activity in bee products: A review. Antioxidants 2021, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Geana, E.I.; Ciucure, C.T. Establishing authenticity of honey via comprehensive Romanian honey analysis. Food Chem. 2020, 306, 125595. [Google Scholar] [CrossRef]
- Guler, A.; Kocaokutgen, H.; Garipoglu, A.V.; Onder, H.; Ekinci, D.; Biyik, S. Detection of adulterated honey produced by honeybee (Apis mellifera L.) colonies fed with different levels of commercial industrial sugar (C3 and C4 plants) syrups by the carbon isotope ratio analysis. Food Chem. 2014, 155, 155–160. [Google Scholar] [CrossRef]
- Zhang, Z.; Abdulla, W. On honey authentication and adulterant detection techniques. Food Control 2022, 138, 108992. [Google Scholar] [CrossRef]
- Crane, E. Bees and Beekeeping. Science, Practice and World Resources; Heinemann Newnes: Oxford, UK, 1990; p. 190. [Google Scholar]
- Zábrodská, B.; Vorlová, L. Adulteration of honey and available methods for detection—A review. Acta Vet. Brno 2014, 83, 85–102. [Google Scholar] [CrossRef]
- Se, K.W.; Wahabb, R.A.; Syed Yaacob, S.N.; Ghoshald, S.K. Detection techniques for adulterants in honey: Challenges and recent trends. J. Food Compos. Anal. 2019, 80, 16–32. [Google Scholar] [CrossRef]
- AOAC Official Method 978.17. In Corn and Cane Sugar Products in Honey; AOAC Official Methods of Analysis. Sugars and Sugar Products; AOAC: Arlington, VA, USA, 1995; Chapter 44; pp. 27–29.
- AOAC Official Method 991.41. In C4 Plant Sugars in Honey; AOAC Official Methods of Analysis. Sugars and Sugar Products; AOAC: Arlington, VA, USA, 1995; Chapter 44; pp. 29–31.
- De Souza, R.R.; Fernandes, D.D.D.; Diniz, P.H.G.D. Honey authentication in terms of its adulteration with sugar syrups using UV-Vis spectroscopy and one-class classifiers. Food Chem. 2021, 365, 130467. [Google Scholar] [CrossRef] [PubMed]
- Valinger, D.; Longin, L.; Grbeš, F.; Benković, M.; Jurina, T.; Gajdoš Kljusurić, J.; Tušek, A.J. Detection of honey adulteration—The potential of UV-VIS and NIR spectroscopy coupled with multivariate analysis. LWT-Food Sci. Technol. 2021, 145, 111316. [Google Scholar] [CrossRef]
- Ali, H.; Khan, S.; Ullah, R.; Khan, B. Fluorescence fingerprints of Sidr honey in comparison with uni/polyfloral honey samples. Eur. Food Res. Technol. 2020, 246, 1829–1837. [Google Scholar] [CrossRef]
- Hao, S.Y.; Li, J.Y.; Liu, X.Y.; Yuan, J.; Yuan, W.Q.; Tian, Y.; Xuan, H.Z. Authentication of acacia honey using fluorescence spectroscopy. Food Control 2021, 130, 108327. [Google Scholar] [CrossRef]
- Megherbi, M.; Herbreteau, B.; Faure, R.; Salvador, A. Polysaccharides as a marker for detection of corn sugar syrup addition in honey. J. Agric. Food Chem. 2009, 57, 2105–2111. [Google Scholar] [CrossRef]
- Du, B.; Wu, L.; Xue, X.; Chen, L.; Li, Y.; Zhao, J.; Cao, W. Rapid Screening of Multiclass Syrup Adulterants in Honey by Ultrahigh-Performance Liquid Chromatography/Quadrupole Time of Flight Mass Spectrometry. J. Agric. Food Chem. 2015, 63, 6614–6623. [Google Scholar] [CrossRef]
- Luong, D.V.; Tam, N.Q.; Xuan, D.T.T.; Tai, N.T. NMR based metabolomic approach for evaluation of Vietnamese honey. Vietnam J. Chem. 2019, 6, 712–716. [Google Scholar] [CrossRef]
- Vasić, V.; Đurđić, S.; Tosti, T.; Radoičić, A.; Lušić, D.; Milojković-Opsenica, D.; Tešić, Ž.; Trifković, J. Two aspects of honeydew honey authenticity: Application of advance analytical methods and chemometrics. Food Chem. 2020, 305, 125457. [Google Scholar] [CrossRef]
- Brendel, R.; Schwolow, S.; Gerhardt, N.; Schwab, J.; Rau, P.; Oest, M.; Rohn, S.; Weller, P. MIR spectroscopy versus MALDI-ToF-MS for authenticity control of honeys from different botanical origins based on soft independent modelling by class analogy (SIMCA)—A clash of techniques? Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 263, 120225. [Google Scholar] [CrossRef]
- Bachmann, R.; Horns, A.L.; Paasch, N.; Schrieck, R.; Weidner, M.; Fransson, I.; Schrör, J. Minor metabolites as chemical marker for the differentiation of cane, beet and coconut blossom sugar. From profiling towards identification of adulterations. Food Control 2022, 135, 108832. [Google Scholar] [CrossRef]
- Cubero-Leon, E.; Peñalver, R.; Maquet, A. Review on metabolomics for food authentication. Food Res. Int. 2014, 60, 95–107. [Google Scholar] [CrossRef]
- EU. Regulation (EC) No 767/2009 of the European Parliament and of the Council of 13 July 2009 on the Placing on the Market and Use of Feed, Amending European Parliament and Council Regulation (EC) No 1831/2003 and Repealing Council Directive 79/373/EEC, Commission Directive 80/511/EEC, Council Directives 82/471/EEC, 83/228/EEC, 93/74/EEC, 93/113/EC and 96/25/EC and Commission Decision 2004/217/EC. OJ L229, 1.09.2009, 1–28. 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32009R0767 (accessed on 12 August 2022).
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Want, E.J.; Masson, P.; Michopoulos, P.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Song, M.; Wang, K.; Fang, X.; Peng, W.; Wu, L.; Xue, X. Detection of acacia honey adulteration with high fructose corn syrup through determination of targeted α Dicarbonyl compound using ion mobility-mass spectrometry coupled with UHPLC-MS/MS. Food Chem. 2021, 352, 129312. [Google Scholar] [CrossRef]
- Ruiz-Matute, A.I.; Soria, A.C.; Martínez-Castro, I.; Sanz, M.L. A new methodology based on GC-MS to detect honey adulteration with commercial syrups. J. Agric. Food Chem. 2007, 55, 7264–7269. [Google Scholar] [CrossRef]
- Ruiz-Matute, A.I.; Rodríguez-Sánchez, S.; Sanz, M.L.; Martínez-Castro, I. Detection of adulterations of honey with high fructose syrups from inulin by GC analysis. J. Food Compos. Anal. 2010, 23, 273–276. [Google Scholar] [CrossRef]
- Bogdanov, S.; Ruoff, K.; Persano Oddo, L. Physico-chemical methods for the characterization of unifloral honeys: A review. Apidologie 2004, 35 (Suppl. S1), S4–S17. [Google Scholar] [CrossRef] [Green Version]
- Fakhlaei, R.; Selamat, J.; Khatib, A.; Razis, A.F.A.; Sukor, R.; Ahmad, S.; Babadi, A.A. The Toxic Impact of Honey Adulteration: A Review. Foods 2020, 9, 1538. [Google Scholar] [CrossRef]
- Morales, V.; Corzo, N.; Sanz, M.L. HPAEC-PAD oligosaccharide analysis to detect adulterations of honey with sugar syrups. Food Chem. 2008, 107, 922–928. [Google Scholar] [CrossRef]
- Waheed Iqbal, M.; Riaz, T.; Hassanin, H.A.M.; Zhang, W.; Saeed, M.; Mahmood, S.; Abdalla, M.; Mu, W. Biochemical characterization of recombinant L-fucose isomerase from Caldanaerobius polysaccharolyticus for L-fuculose production. Int. J. Biol. Macromol. 2020, 146, 965–975. [Google Scholar] [CrossRef]
- Vanhanen, L.P.; Emmertz, A.; Savage, G.P. Mineral analysis of mono-floral New Zealand honey. Food Chem. 2011, 128, 236–240. [Google Scholar] [CrossRef] [PubMed]



| Botanical Origin | Fructose (%) | Glucose (%) | Disaccharides (%) | |
|---|---|---|---|---|
| honey 1 | 38 | 31 | 12 | |
| syrup 1 | sugar beet | 39 | 31 | 30 |
| syrup 2 | sugar beet | 41–44 | 31–35 | 23–26 |
| syrup 3 | sugar beet and corn | 42–46 | 23–28 | 13–19 |
| syrup 4 | sugar beet | 43 | 32 | 25 |
| syrup 5 | sugar beet and corn | 45 | 24 | 15 |
| syrup 6 | corn | 42 | n.d. | n.d. |
| syrup 7 | wheat | 32 | 26 | n.d. |
| syrup 8 | wheat | 43–47 | 20–26 | 15 |
| syrup 9 | wheat | 31–36 | 43–49 | 11–16 |
| syrup 10 | wheat | 17–20 | 26–30 | 33–39 |
| m/z | Retention Time (min) | Ion Mode | Adduct | Molecular Formula | Molecular Weight | Annotation | Ratio (Syrup/Honey) | p-Value |
|---|---|---|---|---|---|---|---|---|
| 363.0681 | 3.31 | positive | [M+K]+ | C12H20O10 | 324.1057 | bis-D-fructose 2′,1:2,1′-dianhydride | 13.8 | 4.20 × 10−4 |
| 504.1916 | 2.55 | positive | [M+NH4-H2O]+ | C18H32O16 | 504.1690 | maltotriose | 14.6 | 2.78 × 10−4 |
| 347.0941 | 3.31 | positive | [M+Na]+ | C12H20O10 | 324.1057 | bis-D-fructose 2′,1:2,1′-dianhydride | 15.3 | 1.01 × 10−3 |
| 316.2114 | 1.27 | positive | [M+H]+ | - | 315.2038 | - | 21.3 | 5.49 × 10−12 |
| 344.5875 | 2.55 | positive | - | - | - | - | 23.6 | 1.14 × 10−3 |
| 316.2114 | 1.05 | positive | [M+H]+ | - | 315.2038 | - | 23.9 | 4.68 × 10−9 |
| 515.1444 | 3.31 | positive | [2M-H2O+H+K]++ | C25H28O11 | 504.1626 | - | 69.5 | 6.65 × 10−4 |
| 243.0274 | 1.23 | negative | [M-H]− | C6H13O8P | 244.0348 | fuculose 1-phosphate | 10.1 | 3.52 × 10−4 |
| 270.9254 | 7.16 | negative | - | - | - | - | 12.5 | 5.18 × 10−5 |
| 245.0244 | 1.24 | negative | [M-H]− | C8H12N2O3P2 | 246.0316 | - | 14.1 | 4.60 × 10−4 |
| 270.0468 | 1.24 | negative | [M-H]− | C7H13NO10 | 271.0540 | - | 15.5 | 2.44 × 10−4 |
| 194.0298 | 0.93 | negative | [M-H]− | C5H9NO7 | 195.0371 | - | 17.2 | 7.47 × 10−4 |
| 183.0290 | 1.64 | negative | - | - | - | - | 39.2 | 2.62 × 10-4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinello, M.; Stella, R.; Baggio, A.; Biancotto, G.; Mutinelli, F. LC-HRMS-Based Non-Targeted Metabolomics for the Assessment of Honey Adulteration with Sugar Syrups: A Preliminary Study. Metabolites 2022, 12, 985. https://doi.org/10.3390/metabo12100985
Martinello M, Stella R, Baggio A, Biancotto G, Mutinelli F. LC-HRMS-Based Non-Targeted Metabolomics for the Assessment of Honey Adulteration with Sugar Syrups: A Preliminary Study. Metabolites. 2022; 12(10):985. https://doi.org/10.3390/metabo12100985
Chicago/Turabian StyleMartinello, Marianna, Roberto Stella, Alessandra Baggio, Giancarlo Biancotto, and Franco Mutinelli. 2022. "LC-HRMS-Based Non-Targeted Metabolomics for the Assessment of Honey Adulteration with Sugar Syrups: A Preliminary Study" Metabolites 12, no. 10: 985. https://doi.org/10.3390/metabo12100985
APA StyleMartinello, M., Stella, R., Baggio, A., Biancotto, G., & Mutinelli, F. (2022). LC-HRMS-Based Non-Targeted Metabolomics for the Assessment of Honey Adulteration with Sugar Syrups: A Preliminary Study. Metabolites, 12(10), 985. https://doi.org/10.3390/metabo12100985