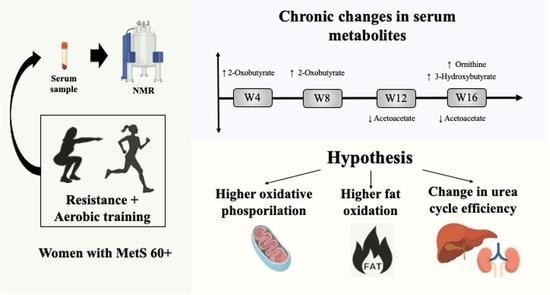
Metabolomic Response throughout 16 Weeks of Combined Aerobic and Resistance Exercise Training in Older Women with Metabolic Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Protocol
2.3. Body Composition
2.4. Cardiorespiratory Fitness
2.5. Blood Pressure
2.6. Blood Measures
2.7. Blood Sample Preparation for Metabolomics Analysis
2.8. NMR Spectrum Acquisition and Metabolite Quantification
2.9. Additional Assessments
2.10. Exercise Training Protocol
2.11. Statistical Analysis
3. Results
3.1. Cardiorespiratory Fitness and RT Load
3.2. Metabolomic Response to Exercise
4. Discussion
Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walls, H.L.; Backholer, K.; Proietto, J.; McNeil, J. Obesity and Trends in Life Expectancy. J. Obes. 2012, 2012, 107989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preston, S.H.; Stokes, A. Contribution of obesity to international differences in life expectancy. Am. J. Public Health 2011, 101, 2137–2143. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bechtold, M.; Palmer, J.; Valtos, J.; Iasiello, C.; Sowers, J. Metabolic syndrome in the elderly. Curr. Diab. Rep. 2006, 6, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Khajedaluee, M.; Hassannia, T.; Rezaee, A.; Ziadi, M.; Dadgarmoghaddam, M. The prevalence of hypertension and its relationship with demographic factors, biochemical, and anthropometric indicators: A population-based study. ARYA Atheroscler. 2016, 12, 259. [Google Scholar]
- Lionakis, N.; Mendrinos, D.; Sanidas, E.; Favatas, G.; Georgopoulou, M. Hypertension in the elderly. World J. Cardiol. 2012, 4, 135. [Google Scholar] [CrossRef]
- Lacruz, M.E.; Kluttig, A.; Hartwig, S.; Löer, M.; Tiller, D.; Greiser, K.H.; Werdan, K.; Haerting, J. Prevalence and Incidence of Hypertension in the General Adult Population. Medicine 2015, 94, e952. [Google Scholar] [CrossRef]
- Ostman, C.; Smart, N.A.; Morcos, D.; Duller, A.; Ridley, W.; Jewiss, D. The effect of exercise training on clinical outcomes in patients with the metabolic syndrome: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2017, 16, 110. [Google Scholar] [CrossRef] [Green Version]
- Duft, R.G.; Castro, A.; Bonfante, I.L.P.; Brunelli, D.T.; Chacon-Mikahil, M.P.T.; Cavaglieri, C.R. Metabolomics Approach in the Investigation of Metabolic Changes in Obese Men after 24 Weeks of Combined Training. J. Proteome Res. 2017, 16, 2151–2159. [Google Scholar] [CrossRef]
- Castro, A.; Duft, R.G.; Ferreira, M.L.V.; De Andrade, A.L.L.; Gáspari, A.F.; Silva, L.D.M.; De Oliveira-Nunes, S.G.; Cavaglieri, C.R.; Ghosh, S.; Bouchard, C.; et al. Association of skeletal muscle and serum metabolites with maximum power output gains in response to continuous endurance or high-intensity interval training programs: The TIMES study—A randomized controlled trial. PLoS ONE 2019, 14, e0212115. [Google Scholar] [CrossRef] [Green Version]
- Brennan, A.M.; Benson, M.; Morningstar, J.; Herzig, M.; Robbins, J.; Gerszten, R.E.; Ross, R. Plasma Metabolite Profiles in Response to Chronic Exercise. Med. Sci. Sports Exerc. 2018, 50, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Arend, E.; Rocha, S.M.; Rudnitskaya, A.; Delgado, L.; Moreira, A.; Carvalho, J. The impact of exercise training on the lipid peroxidation metabolomic profile and respiratory infection risk in older adults. Eur. J. Sport Sci. 2019, 19, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Wilson, I.D. Opinion: Understanding “global” systems biology: Metabonomics and the continuum of metabolism. Nat. Rev. Drug Discov. 2003, 2, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Metabolomics: The principles and potential applications to transplantation. Am. J. Transplant. 2005, 5, 2814–2820. [Google Scholar] [CrossRef]
- Kelly, R.S.; Kelly, M.P.; Kelly, P. Metabolomics, physical activity, exercise and health: A review of the current evidence. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 1–17. [Google Scholar] [CrossRef]
- Khoramipour, K.; Sandbakk, Ø.; Keshteli, A.H.; Gaeini, A.A.; Wishart, D.S.; Chamari, K. Metabolomics in Exercise and Sports: A Systematic Review. Sports Med. 2022, 52, 547–583. [Google Scholar] [CrossRef]
- Castro, A.; Duft, R.G.; de Oliveira-Nunes, S.G.; de Andrade, A.L.L.; Cavaglieri, C.R.; Chacon-Mikahil, M.P.T. Association Between Changes in Serum and Skeletal Muscle Metabolomics Profile With Maximum Power Output Gains in Response to Different Aerobic Training Programs: The Times Study. Front. Physiol. 2021, 12, 1–17. [Google Scholar] [CrossRef]
- Santilli, F.; D’Ardes, D.; Guagnano, M.T.; Davi, G. Metabolic Syndrome: Sex-Related Cardiovascular Risk and Therapeutic Approach. Curr. Med. Chem. 2017, 24, 2602–2627. [Google Scholar] [CrossRef]
- Jansson, A.; Gunnarsson, V.; Ringmark, S.; Ragnarsson, S.; Söderroos, D.; Ásgeirsson, E.; Jóhannsdóttir, T.R.; Liedberg, C.; Stefánsdóttir, G.J. Increased body fat content in horses alters metabolic and physiological exercise response, decreases performance, and increases locomotion asymmetry. Physiol. Rep. 2021, 9, e14824. [Google Scholar] [CrossRef]
- Kistner, S.; Döring, M.; Krüger, R.; Rist, M.; Weinert, C.; Bunzel, D.; Merz, B.; Radloff, K.; Neumann, R.; Härtel, S.; et al. Sex-Specific Relationship between the Cardiorespiratory Fitness and Plasma Metabolite Patterns in Healthy Humans-Results of the KarMeN Study. Metabolites 2021, 11, 463. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Koal, T.; Wang, Y.; Kohl, M.; Enot, D.P.; Deigner, H.P. Targeted metabolomics for biomarker discovery. Angew. Chem. Int. Ed. Engl. 2010, 49, 5426–5445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sun, H.; Wang, X. Power of metabolomics in biomarker discovery and mining mechanisms of obesity. Obes. Rev. 2013, 14, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Sardeli, A.V.; Gáspari, A.F.; dos Santos, W.M.; de Araujo, A.A.; de Angelis, K.; Mariano, L.O.; Cavaglieri, C.R.; Fernhall, B.; Chacon-Mikahil, M.P.T. Comprehensive Time-Course Effects of Combined Training on Hypertensive Older Adults: A Randomized Control Trial. Int. J. Environ. Res. Public Health 2022, 19, 11042. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- McCrory, M.A.; Gomez, T.D.; BeRnauer, E.M.; Mole, P.A. Evaluation of a new air displacement plethysmograph for measuring human body composition. Med. Sci. Sports Exerc. 1995, 27, 1686–1691. [Google Scholar] [CrossRef] [Green Version]
- Siri, W. Body composition from fluid spaces and density: Analysis of methods. 1961. Nutrition 1993, 9, 480–491. [Google Scholar]
- Sardeli, A.; Gáspari, A.; Dos Santos, W.; Moraes, D.F.G.; Gadelha, V.B.; do Santos, L.C.; Ferreira, M.L.V.; de Prudencio, S.M.J.; Bonfnate, I.L.P.; Rodrigues, B.; et al. Time-course of health-related adaptations in response to combined training in hypertensive elderly: Immune and autonomic modulation interactions. Mot. Rev. Educ. Física 2018, 24, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, M.L.V.; Sardeli, A.V.; Souza GVDe Bonganha, V.; Santos, L.D.C.; Castro, A.; Cavaglieri, C.R.; Chacon-Mikahil, M.P.T. Cardiac autonomic and haemodynamic recovery after a single session of aerobic exercise with and without blood flow restriction in older adults. J. Sports Sci. 2017, 35, 2412–2420. [Google Scholar] [CrossRef]
- Howley, E.T.; Bassett, D.R.; Welch, H.G. Criteria for maximal oxygen uptake: Review and commentary. Med. Sci. Sports Exerc. 1995, 27, 1292–1301. [Google Scholar] [CrossRef]
- Azevedo, P.; Bhammar, D.; Babb, T.; Bowen, T.S.; Witte, K.K.; Rossiter, H.B.; Brugniaux, J.V.; Perry, B.D.; Lucas, R.D.; Turnes, T.; et al. Commentaries on Viewpoint: Vo2peak is an acceptable estimate of cardiorespiratory fitness but not Vo2max. J. Appl. Physiol. 2018, 125, 237. [Google Scholar]
- Heubert, R.A.P.; Billat, V.L.; Chassaing, P.; Bocquet, V.; Morton, R.H.; Koralsztein, J.P.; Di Prampero, P.E. Effect of a previous sprint on the parameters of the work-time to exhaustion relationship in high intensity cycling. Int. J. Sports Med. 2005, 26, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Perloff, D.; Grim, C.; Flack, J.; Frohlich, E.D.; Hill, M.; McDonald, M.; Morgenstern, B.Z. Human blood pressure determination by sphygmomanometry. Circulation 1993, 88, 2460–2470. [Google Scholar] [CrossRef] [PubMed]
- Guelen, I.; Westerhof, B.E.; Van Der Sar, G.L.; Van Montfrans, G.A.; Kiemeneij, F.; Wesseling, K.H.; Bos, W.J.W. Validation of brachial artery pressure reconstruction from finger arterial pressure. J. Hypertens. 2008, 26, 1321–1327. [Google Scholar] [CrossRef]
- Sardeli, A.V.; Santos, L.C.; Ferreira, M.L.V.; Gáspari, A.F.; Rodrigues, B.; Cavaglieri, C.R.; Chacon-Mikahil, M.P.T. Cardiovascular Responses to Different resistance Exercise protocols in Elderly. Int. J. Sports Med. 2017, 38, 928–936. [Google Scholar] [CrossRef]
- Lane, A.N. The Handbook of Metabolomics, 17th ed.; Humana Press: Totowa, NJ, USA, 2012. [Google Scholar]
- Sardeli, A.V.; Bellotto, M.L.; Moraes, D.F.G.; Santos, W.M.; Gadelha, V.B.; Ferreira, M.L.V.; Chacon-Miikahil, M.P.T. Influence of physical training on the food choices of elderly individuals. Mundo Saúde 2020, 44, 300–310. [Google Scholar] [CrossRef]
- Chodzko-Zajko, W.J.; Proctor, D.N.; Fiatarone Singh, M.A.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. Exercise and Physical Activity for Older Adults. Med. Sci. Sport Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance Training for Older Adults: Position Statement From the National Strength and Conditioning Association. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef]
- Pescatello, L.S.; Franklin, B.A.; Fagard, R.; Farquhar, W.B.; Kelley, G.A.; Ray, C.A.; American College of Sports Medicine. American College of Sports Medicine position stand. Exercise and hypertension. Med. Sci. Sports Exerc. 2004, 36, 533–553. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P.; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- van den Berg, R.A.; Hoefsloot, H.C.J.; Westerhuis, J.A.; Smilde, A.K.; van der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genom. 2006, 7, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Earnest, C.P.; Johannsen, N.M.; Swift, D.L.; Gillison, F.B.; Mikus, C.R.; Lucia, A.; Kramer, K.; Lavie, C.J.; Church, T.S. Aerobic and strength training in concomitant metabolic syndrome and type 2 diabetes. Med. Sci. Sports Exerc. 2014, 46, 1293–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badr, H.E.; Saunders, T.; Carter, A.; Reyes Castillo, L.; Bayoumy, O.; Barrett, M. Impact of Lifestyle Modification on Quality of Life in Patients with Metabolic Syndrome: Findings from the CHANGE Program Intervention Study in Prince Edward Island, Canada. Metab. Syndr. Relat. Disord 2022. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, C.A.; Nieman, D.C.; Signini, E.F.; Abreu, R.M.; Catai, A.M. Metabolomics-Based Studies Assessing Exercise-Induced Alterations of the Human Metabolome: A Systematic Review. Metabolites 2019, 9, 164. [Google Scholar] [CrossRef] [Green Version]
- Sugino, T.; Shirai, T.; Kajimoto, Y.; Kajimoto, O. L-Ornithine supplementation attenuates physical fatigue in healthy volunteers by modulating lipid and amino acid metabolism. Nutr. Res. 2008, 28, 738–743. [Google Scholar] [CrossRef]
- Demura, S.; Morishita, K.; Yamada, T.; Yamaji, S.; Komatsu, M. Effect of L-ornithine hydrochloride ingestion on intermittent maximal anaerobic cycle ergometer performance and fatigue recovery after exercise. Eur. J. Appl. Physiol. 2011, 111, 2837–2843. [Google Scholar] [CrossRef] [Green Version]
- Miyake, M.; Kirisako, T.; Kokubo, T.; Miura, Y.; Morishita, K.; Okamura, H.; Tsuda, A. Randomised controlled trial of the effects of L-ornithine on stress markers and sleep quality in healthy workers. Nutr. J. 2014, 13, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Meyer, T.W.; Hostetter, T.H. Uremia. N. Engl. J. Med. 2007, 357, 1316–1325. [Google Scholar] [CrossRef]
- Ivanovski, I.; Ješić, M.; Ivanovski, A.; Garavelli, L.; Ivanovski, P. Metabolically based liver damage pathophysiology in patients with urea cycle disorders—A new hypothesis. World J. Gastroenterol. 2017, 23, 7930. [Google Scholar] [CrossRef]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef]
- Tarracchini, C.; Fontana, F.; Lugli, G.A.; Mancabelli, L.; Alessandri, G.; Turroni, F.; Ventura, M.; Milani, C. Investigation of the Ecological Link between Recurrent Microbial Human Gut Communities and Physical Activity. Microbiol. Spectr. 2022, 10, e00420-22. [Google Scholar] [CrossRef] [PubMed]
- Dandanell, S.; Meinild-Lundby, A.K.; Andersen, A.B.; Lang, P.F.; Oberholzer, L.; Keiser, S.; Robach, P.; Larsen, S.; Rønnestad, B.R.; Lundby, C. Determinants of maximal whole-body fat oxidation in elite cross-country skiers: Role of skeletal muscle mitochondria. Scand. J. Med. Sci. Sports 2018, 28, 2494–2504. [Google Scholar] [CrossRef] [PubMed]
- Hetlelid, K.J.; Plews, D.J.; Herold, E.; Laursen, P.B.; Seiler, S. Rethinking the role of fat oxidation: Substrate utilisation during high-intensity interval training in well-trained and recreationally trained runners. BMJ Open Sport Exerc. Med. 2015, 1, e000047. [Google Scholar] [CrossRef] [PubMed]
- Yeo, W.K.; Carey, A.L.; Burke, L.; Spriet, L.L.; Hawley, J.A. Fat adaptation in well-trained athletes: Effects on cell metabolism. Appl. Physiol. Nutr. Metab. 2011, 36, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.F.L.; Muñoz, V.R.; Junqueira, R.L.; de Oliveira, F.; Gaspar, R.C.; Nakandakari, S.C.B.R.; de Oliveira Costa, S.; Torsoni, M.A.; da Silva, A.S.R.; Cintra, D.E.; et al. Time-restricted feeding combined with aerobic exercise training can prevent weight gain and improve metabolic disorders in mice fed a high-fat diet. J. Physiol. 2022, 600, 797–813. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.C.; Verdin, E. β-Hydroxybutyrate: A Signaling Metabolite. Annu. Rev. Nutr. 2017, 37, 51. [Google Scholar] [CrossRef]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G.; Leeuwenburgh, C.; Mattson, M.P. Flipping the Metabolic Switch: Understanding and Applying Health Benefits of Fasting. Obesity 2018, 26, 254. [Google Scholar] [CrossRef] [Green Version]
- Koay, Y.C.; Stanton, K.; Kienzle, V.; Li, M.; Yang, J.; Celermajer, D.S.; O’Sullivan, J.F. Effect of chronic exercise in healthy young male adults: A metabolomic analysis. Cardiovasc. Res. 2021, 117, 613–622. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.-B.; Zhang, Y.-C.; Huang, H.-H.; Lin, J. Prospects for clinical applications of butyrate-producing bacteria. World J. Clin. Pediatr. 2021, 10, 84–92. [Google Scholar] [CrossRef]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The Human Metabolome Database. Nucleic Acids Res. 2007, 35, D521–D526. [Google Scholar] [CrossRef]
- Bay, M.L.; Pedersen, B.K. Muscle-Organ Crosstalk: Focus on Immunometabolism. Front. Physiol. 2020, 11, 567881. [Google Scholar] [CrossRef] [PubMed]
- Von Ah Morano, A.E.; Dorneles, G.P.; Peres, A.; Lira, F.S. The role of glucose homeostasis on immune function in response to exercise: The impact of low or higher energetic conditions. J. Cell. Physiol. 2020, 235, 3169–3188. [Google Scholar] [CrossRef]
- Rosa-Neto, J.C.; Lira, F.S.; Little, J.P.; Landells, G.; Islam, H.; Chazaud, B.; Pyne, D.B.; Teixeira, A.M.; Batatinha, H.; Moura Antunes, B.; et al. Immunometabolism-fit: How exercise and training can modify T cell and macrophage metabolism in health and disease. Exerc. Immunol. Rev. 2022, 28, 29–46. [Google Scholar] [PubMed]
- Padilha, C.S.; Figueiredo, C.; Minuzzi, L.G.; Chimin, P.; Deminice, R.; Krüger, K.; Rosa-Neto, J.C.; Lira, F.S. Immunometabolic responses according to physical fitness status and lifelong exercise during aging: New roads for exercise immunology. Ageing Res. Rev. 2021, 68, 101341. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.; Duft, R.G.; de Mattos Zeri, A.C.; Cavaglieri, C.R.; Chacon-Mikahil, M.P.T. Commentary: Metabolomics-Based Studies Assessing Exercise-Induced Alterations of the Human Metabolome: A Systematic Review. Front. Physiol. 2020, 11, 353. [Google Scholar] [CrossRef] [Green Version]
- Monnerie, S.; Comte, B.; Ziegler, D.; Morais, J.A.; Pujos-Guillot, E.; Gaudreau, P. Metabolomic and Lipidomic Signatures of Metabolic Syndrome and its Physiological Components in Adults: A Systematic Review. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Pujos-Guillot, E.; Brandolini, M.; Pétéra, M.; Grissa, D.; Joly, C.; Lyan, B.; Herquelot, E.; Czernichow, S.; Zins, M.; Goldberg, M.; et al. Systems Metabolomics for Prediction of Metabolic Syndrome. J. Proteome Res. 2017, 16, 2262–2272. [Google Scholar] [CrossRef]
- Barber, J.L.; Ruiz-Ramie, J.J.; Robbins, J.M.; Gerszten, R.E.; Leon, A.S.; Rao, D.C.; Skinner, J.S.; Bouchard, C.; Sarzynski, M.A. Regular exercise and patterns of response across multiple cardiometabolic traits: The HERITAGE family study. Br. J. Sports Med. 2022, 56, 95–100. [Google Scholar] [CrossRef]
- Sardeli, A.V.; Griffth, G.J.; Santos MVMA dos Ito, M.S.R.; Chacon-Mikahil, M.P.T. The effects of exercise training on hypertensive older adults: An umbrella meta-analysis. Hypertens. Res. 2021, 44, 1434–1443. [Google Scholar] [CrossRef]
- Quiroga, R.; Nistal, E.; Estébanez, B.; Porras, D.; Juarez-Fernandez, M.; Martinez-Florez, S.; Garcia-Mediavilla, M.V.; de Paz, J.A.; Gonzalez-Gallego, J.; Sanchez-Campos, S.; et al. Exercise training modulates the gut microbiota profile and impairs inflammatory signaling pathways in obese children. Exp. Mol. Med. 2020, 52, 1048–1061. [Google Scholar] [CrossRef]
- Duft, R.G.; Castro, A.; Bonfante, I.L.P.; Lopes, W.A.; da Silva, L.R.; Chacon-Mikahil, M.P.T.; Leite, N.; Cavaglieri, C.R. Altered metabolomic profiling of overweight and obese adolescents after combined training is associated with reduced insulin resistance. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]


| CG (n = 13) | CT (n = 12) | |||
|---|---|---|---|---|
| Variables | 0W | 16W | 0W | 16W |
| Age (years) | 65.6 ± 5.6 | - | 64.5 ± 3.9 | - |
| Body mass (kg) | 75.9 ± 8.3 | 76 ± 8.7 | 80.7 ± 6.3 | 80.3 ± 7.1 |
| BMI (kg∙m−2) | 30.5 ± 2.6 | 30 ± 2.7 | 30.3 ± 2.3 | 30.1 ± 2.5 |
| Body fat (%) | 46.3 ± 4.4 | 47.2 ± 4.4 | 45.3 ± 3.5 | 45.1 ± 3.2 |
| Waist circumference (cm) | 103.2 ± 4.8 | 103.2 ± 4.4 | 106.3 ± 5.7 | 103.2 ± 6.5 |
| Systolic BP (mm Hg) | 132.1 ± 27.9 | 125.5 ± 20.1 | 133.8 ± 21.3 | 126 ± 11.9 |
| Diastolic BP (mm Hg) | 84.9 ± 18 | 78.1 ± 9.2 | 84.6 ± 10.8 | 82.5 ± 9.1 |
| Glycemia (mg∙dL−1) | 101.9 ± 13.3 | 97.4 ± 9.9 | 109.7 ± 27.7 | 109.1 ± 25.8 |
| Triglycerides (mg∙dL−1) | 127.2 ± 48.8 | 123.1 ± 48.1 | 105.7 ± 45.9 | 124.5 ± 47.1 |
| Total cholesterol (mg∙dL−1) | 170.8 ± 47.0 | 173.9 ± 50.4 | 174.6 ± 39.1 | 178.2 ± 46.8 |
| HDL-cholesterol (mg∙dL−1) | 41.0 ± 11.6 | 42.5 ± 15.2 | 41.2 ± 12.1 | 43.6 ± 21.6 |
| LDL-cholesterol (mg∙dL−1) | 105.2 ± 38.1 | 106.7 ± 39.1 | 112.2 ± 34.0 | 109.7 ± 32.9 |
| CRP (mg∙L−1) | 2 ± 0.4 | 1.5 ± 0.6 | 1.8 ± 0.5 | 1.3 ± 0.3 |
| TNF-α (pg∙mL−1) | 1.4 ± 0.2 | 1.6 ± 0.2 | 1.5 ± 0.1 | 1.8 ± 0.3 |
| RT Load (kg) | W0 | W4 | W8 | W12 | W16 |
|---|---|---|---|---|---|
| Leg extension | 11.73 ± 2.2 | 17.73 ± 3.9 a | 21 ± 4.9 a | 25.45 ± 4.7 ab | 27.18 ± 5.1 abc |
| Leg flexion | 11.73 ± 3 | 16.64 ± 2.9 | 20.55 ± 5.4 a | 22.27 ± 4.4 ab | 22.64 ± 5.1 ab |
| Leg press | 23.64 ± 10.3 | 37.27 ± 7.9 | 50 ± 12.6 a | 61.82 ± 10.8 ab | 64.55 ± 11.3 abc |
| Bench press | 3.18 ± 1.3 | 4.64 ± 1.4 | 6.09 ± 1.2 a | 6.45 ± 1.4 a | 6.64 ± 1.4 ab |
| Lat pulldown | 15.45 ± 2.7 | 19.09 ± 3.8 | 20.91 ± 4.4 a | 22.27 ± 4.1 a | 23.64 ± 4.5 a |
| Metabolite | CT (mM) | ||||
| 0W | 4W | 8W | 12W | 16W | |
| 2-Oxobutyrate | 0.0029 ± 0.0009 | 0.0038 ± 0.0011 a | 0.0041 ± 0.0015 a | 0.0038 ± 0.0012 | 0.0034 ± 0.0011 |
| 3-Hydroxybutyrate | 0.0717 ± 0.0377 | 0.0483 ± 0.0421 | 0.0420 ± 0.0219 | 0.0372 ± 0.0186 | 0.0397 ± 0.0331 a |
| Acetoacetate | 0.0441 ± 0.0240 | 0.0299 ± 0.0214 | 0.0268 ± 0.0127 | 0.0224 ± 0.0071 a | 0.0239 ± 0.0141 a |
| Ornithine | 0.0100 ± 0.0028 | 0.0110 ± 0.0025 | 0.0098 ± 0.0036 | 0.0105 ± 0.0030 | 0.0182 ± 0.0152 b |
| CG (mM) | |||||
| 0W | 4W | 8W | 12W | 16W | |
| 2-Oxobutyrate | 0.0026 ± 0.0009 | 0.0026 ± 0.0010 | 0.0028 ± 0.0007 | 0.0026 ± 0.0009 | 0.0032 ± 0.0012 |
| 3-Hydroxybutyrate | 0.0437 ± 0.0384 | 0.0275 ± 0.0111 | 0.0387 ± 0.0317 | 0.0475 ± 0.0598 | 0.0523 ± 0.0361 |
| Acetoacetate | 0.0260 ± 0.0188 | 0.0196 ± 0.0068 | 0.0220 ± 0.0148 | 0.0261 ± 0.0260 | 0.0277 ± 0.0136 |
| Ornithine | 0.0116 ± 0.0050 | 0.0110 ± 0.0047 | 0.0104 ± 0.0038 | 0.0117 ± 0.0046 | 0.0102 ± 0.0041 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sardeli, A.V.; Castro, A.; Gadelha, V.B.; Santos, W.M.d.; Lord, J.M.; Cavaglieri, C.R.; Chacon-Mikahil, M.P.T. Metabolomic Response throughout 16 Weeks of Combined Aerobic and Resistance Exercise Training in Older Women with Metabolic Syndrome. Metabolites 2022, 12, 1041. https://doi.org/10.3390/metabo12111041
Sardeli AV, Castro A, Gadelha VB, Santos WMd, Lord JM, Cavaglieri CR, Chacon-Mikahil MPT. Metabolomic Response throughout 16 Weeks of Combined Aerobic and Resistance Exercise Training in Older Women with Metabolic Syndrome. Metabolites. 2022; 12(11):1041. https://doi.org/10.3390/metabo12111041
Chicago/Turabian StyleSardeli, Amanda V., Alex Castro, Victor B. Gadelha, Wellington M. dos Santos, Janet M. Lord, Cláudia R. Cavaglieri, and Mara Patrícia T. Chacon-Mikahil. 2022. "Metabolomic Response throughout 16 Weeks of Combined Aerobic and Resistance Exercise Training in Older Women with Metabolic Syndrome" Metabolites 12, no. 11: 1041. https://doi.org/10.3390/metabo12111041
APA StyleSardeli, A. V., Castro, A., Gadelha, V. B., Santos, W. M. d., Lord, J. M., Cavaglieri, C. R., & Chacon-Mikahil, M. P. T. (2022). Metabolomic Response throughout 16 Weeks of Combined Aerobic and Resistance Exercise Training in Older Women with Metabolic Syndrome. Metabolites, 12(11), 1041. https://doi.org/10.3390/metabo12111041