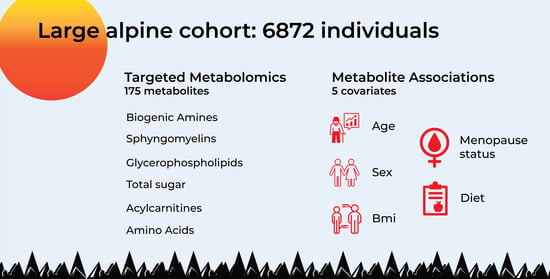
Age, Sex, Body Mass Index, Diet and Menopause Related Metabolites in a Large Homogeneous Alpine Cohort
Abstract
:1. Introduction
2. Results
2.1. Study Sample Characteristics and General Data Overview
2.2. Sex, Age and Body Mass Index-Related Metabolites
2.3. Menopause-Related Metabolites
2.4. Metabolites Related to Food Items and Food Groups
3. Discussion
3.1. Sex, Age and Body Mass Index-Related Metabolites
3.2. Menopause-Related Metabolites
3.3. Metabolites Related to Food Items and Food Groups
4. Materials and Methods
4.1. Participant Recruitment and Sample Collection
4.2. Sample Preparation and Data Acquisition
4.3. Data Preprocessing
4.4. Statistical Analysis
4.5. Comparison of Significant Metabolites with Results from Literature
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lasky-Su, J.; Kelly, R.S.; Wheelock, C.E.; Broadhurst, D. A Strategy for Advancing for Population-Based Scientific Discovery Using the Metabolome: The Establishment of the Metabolomics Society Metabolomic Epidemiology Task Group. Metabolomics 2021, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- Van Roekel, E.H.; Loftfield, E.; Kelly, R.S.; Zeleznik, O.A.; Zanetti, K.A. Metabolomics in Epidemiologic Research: Challenges and Opportunities for Early-Career Epidemiologists. Metab. Off. J. Metab. Soc. 2019, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- Rist, M.J.; Roth, A.; Frommherz, L.; Weinert, C.H.; Krüger, R.; Merz, B.; Bunzel, D.; Mack, C.; Egert, B.; Bub, A.; et al. Metabolite Patterns Predicting Sex and Age in Participants of the Karlsruhe Metabolomics and Nutrition (KarMeN) Study. PLoS ONE 2017, 12, e0183228. [Google Scholar] [CrossRef]
- Vignoli, A.; Tenori, L.; Luchinat, C.; Saccenti, E. Age and Sex Effects on Plasma Metabolite Association Networks in Healthy Subjects. J. Proteome Res. 2018, 17, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Carayol, M.; Leitzmann, M.F.; Ferrari, P.; Zamora-Ros, R.; Achaintre, D.; Stepien, M.; Schmidt, J.A.; Travis, R.C.; Overvad, K.; Tjønneland, A.; et al. Blood Metabolic Signatures of Body Mass Index: A Targeted Metabolomics Study in the EPIC Cohort. J. Proteome Res. 2017, 16, 3137–3146. [Google Scholar] [CrossRef]
- Caterino, M.; Ruoppolo, M.; Costanzo, M.; Albano, L.; Crisci, D.; Sotgiu, G.; Saderi, L.; Montella, A.; Franconi, F.; Campesi, I. Sex Affects Human Premature Neonates’ Blood Metabolome According to Gestational Age, Parenteral Nutrition, and Caffeine Treatment. Metabolites 2021, 11, 158. [Google Scholar] [CrossRef]
- Chaleckis, R.; Murakami, I.; Takada, J.; Kondoh, H.; Yanagida, M. Individual Variability in Human Blood Metabolites Identifies Age-Related Differences. Proc. Natl. Acad. Sci. USA 2016, 113, 4252–4259. [Google Scholar] [CrossRef] [Green Version]
- Moore, S.C.; Matthews, C.E.; Sampson, J.N.; Stolzenberg-Solomon, R.Z.; Zheng, W.; Cai, Q.; Tan, Y.T.; Chow, W.-H.; Ji, B.-T.; Liu, D.K.; et al. Human Metabolic Correlates of Body Mass Index. Metab. Off. J. Metab. Soc. 2014, 10, 259–269. [Google Scholar] [CrossRef]
- Perng, W.; Rahman, M.L.; Aris, I.M.; Michelotti, G.; Sordillo, J.E.; Chavarro, J.E.; Oken, E.; Hivert, M.-F. Metabolite Profiles of the Relationship between Body Mass Index (BMI) Milestones and Metabolic Risk during Early Adolescence. Metabolites 2020, 10, 316. [Google Scholar] [CrossRef]
- Ottosson, F.; Brunkwall, L.; Ericson, U.; Nilsson, P.M.; Almgren, P.; Fernandez, C.; Melander, O.; Orho-Melander, M. Connection Between BMI-Related Plasma Metabolite Profile and Gut Microbiota. J. Clin. Endocrinol. Metab. 2018, 103, 1491–1501. [Google Scholar] [CrossRef] [Green Version]
- Dunn, W.B.; Lin, W.; Broadhurst, D.; Begley, P.; Brown, M.; Zelena, E.; Vaughan, A.A.; Halsall, A.; Harding, N.; Knowles, J.D.; et al. Molecular Phenotyping of a UK Population: Defining the Human Serum Metabolome. Metab. Off. J. Metab. Soc. 2015, 11, 9–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darst, B.F.; Koscik, R.L.; Hogan, K.J.; Johnson, S.C.; Engelman, C.D. Longitudinal Plasma Metabolomics of Aging and Sex. Aging 2019, 11, 1262–1282. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhai, G.; Singmann, P.; He, Y.; Xu, T.; Prehn, C.; Römisch-Margl, W.; Lattka, E.; Gieger, C.; Soranzo, N.; et al. Human Serum Metabolic Profiles Are Age Dependent. Aging Cell 2012, 11, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Mittelstraß, K.; Ried, J.S.; Yu, Z.; Krumsiek, J.; Gieger, C.; Prehn, C.; Roemisch-Margl, W.; Polonikov, A.; Peters, A.; Theis, F.J.; et al. Discovery of Sexual Dimorphisms in Metabolic and Genetic Biomarkers. PLoS Genet. 2011, 7, e1002215. [Google Scholar] [CrossRef] [Green Version]
- Krumsiek, J.; Mittelstraß, K.; Do, K.T.; Stückler, F.; Ried, J.; Adamski, J.; Peters, A.; Illig, T.; Kronenberg, F.; Friedrich, N.; et al. Gender-Specific Pathway Differences in the Human Serum Metabolome. Metab. Off. J. Metab. Soc. 2015, 11, 1815–1833. [Google Scholar] [CrossRef] [Green Version]
- Ho, J.E.; Larson, M.G.; Ghorbani, A.; Cheng, S.; Chen, M.-H.; Keyes, M.; Rhee, E.P.; Clish, C.B.; Vasan, R.S.; Gerszten, R.E.; et al. Metabolomic Profiles of Body Mass Index in the Framingham Heart Study Reveal Distinct Cardiometabolic Phenotypes. PLoS ONE 2016, 11, e0148361. [Google Scholar] [CrossRef] [Green Version]
- Menni, C.; Kastenmüller, G.; Petersen, A.K.; Bell, J.T.; Psatha, M.; Tsai, P.-C.; Gieger, C.; Schulz, H.; Erte, I.; John, S.; et al. Metabolomic Markers Reveal Novel Pathways of Ageing and Early Development in Human Populations. Int. J. Epidemiol. 2013, 42, 1111–1119. [Google Scholar] [CrossRef] [Green Version]
- Bell, J.A.; Santos Ferreira, D.L.; Fraser, A.; Soares, A.L.G.; Howe, L.D.; Lawlor, D.A.; Carslake, D.; Davey Smith, G.; O’Keeffe, L.M. Sex Differences in Systemic Metabolites at Four Life Stages: Cohort Study with Repeated Metabolomics. BMC Med. 2021, 19, 58. [Google Scholar] [CrossRef]
- Auro, K.; Joensuu, A.; Fischer, K.; Kettunen, J.; Salo, P.; Mattsson, H.; Niironen, M.; Kaprio, J.; Eriksson, J.G.; Lehtimäki, T.; et al. A Metabolic View on Menopause and Ageing. Nat. Commun. 2014, 5, 4708. [Google Scholar] [CrossRef] [Green Version]
- Guasch-Ferré, M.; Bhupathiraju, S.N.; Hu, F.B. Use of Metabolomics in Improving Assessment of Dietary Intake. Clin. Chem. 2018, 64, 82–98. [Google Scholar] [CrossRef] [Green Version]
- Scalbert, A.; Brennan, L.; Manach, C.; Andres-Lacueva, C.; Dragsted, L.O.; Draper, J.; Rappaport, S.M.; van der Hooft, J.J.J.; Wishart, D.S. The Food Metabolome: A Window over Dietary Exposure. Am. J. Clin. Nutr. 2014, 99, 1286–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pattaro, C.; Gögele, M.; Mascalzoni, D.; Melotti, R.; Schwienbacher, C.; De Grandi, A.; Foco, L.; D’Elia, Y.; Linder, B.; Fuchsberger, C.; et al. The Cooperative Health Research in South Tyrol (CHRIS) Study: Rationale, Objectives, and Preliminary Results. J. Transl. Med. 2015, 13, 348. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Han, Q.; Liu, Y.; Sun, C.; Gang, X.; Wang, G. The Relationship between Branched-Chain Amino Acid Related Metabolomic Signature and Insulin Resistance: A Systematic Review. J. Diabetes Res. 2016, 2016, 2794591. [Google Scholar] [CrossRef] [PubMed]
- El-Khoury, J.M.; Bunch, D.R.; Hu, B.; Payto, D.; Reineks, E.Z.; Wang, S. Comparison of Symmetric Dimethylarginine with Creatinine, Cystatin C and Their EGFR Equations as Markers of Kidney Function. Clin. Biochem. 2016, 49, 1140–1143. [Google Scholar] [CrossRef] [PubMed]
- Grace, A.A. Dysregulation of the Dopamine System in the Pathophysiology of Schizophrenia and Depression. Nat. Rev. Neurosci. 2016, 17, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Lawson, M.A.; Kelley, K.W. Inflammation-Associated Depression: From Serotonin to Kynurenine. Psychoneuroendocrinology 2011, 36, 426–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Songtachalert, T.; Roomruangwong, C.; Carvalho, A.F.; Bourin, M.; Maes, M. Anxiety Disorders: Sex Differences in Serotonin and Tryptophan Metabolism. Curr. Top. Med. Chem. 2018, 18, 1704–1715. [Google Scholar] [CrossRef]
- Hodge, S.; Bunting, B.P.; Carr, E.; Strain, J.J.; Stewart-Knox, B.J. Obesity, Whole Blood Serotonin and Sex Differences in Healthy Volunteers. Obes. Facts 2012, 5, 399–407. [Google Scholar] [CrossRef]
- Karrer, T.M.; McLaughlin, C.L.; Guaglianone, C.P.; Samanez-Larkin, G.R. Reduced Serotonin Receptors and Transporters in Normal Aging Adults: A Meta-Analysis of PET and SPECT Imaging Studies. Neurobiol. Aging 2019, 80, 1–10. [Google Scholar] [CrossRef]
- Yamakado, M.; Tanaka, T.; Nagao, K.; Imaizumi, A.; Komatsu, M.; Daimon, T.; Miyano, H.; Tani, M.; Toda, A.; Yamamoto, H.; et al. Plasma Amino Acid Profile Associated with Fatty Liver Disease and Co-Occurrence of Metabolic Risk Factors. Sci. Rep. 2017, 7, 14485. [Google Scholar] [CrossRef] [Green Version]
- Hiraiwa, H.; Okumura, T.; Kondo, T.; Kato, T.; Kazama, S.; Ishihara, T.; Iwata, E.; Shimojo, M.; Kondo, S.; Aoki, S.; et al. Usefulness of the Plasma Branched-Chain Amino Acid/Aromatic Amino Acid Ratio for Predicting Future Cardiac Events in Patients with Heart Failure. J. Cardiol. 2020, 75, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, R.W.; Rodriguez, P.C.; Toque, H.A.; Narayanan, S.P.; Caldwell, R.B. Arginase: A Multifaceted Enzyme Important in Health and Disease. Physiol. Rev. 2018, 98, 641–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goïta, Y.; Chao de la Barca, J.M.; Keïta, A.; Diarra, M.B.; Dembélé, K.C.; Chabrun, F.; Dramé, B.S.I.; Kassogué, Y.; Diakité, M.; Mirebeau-Prunier, D.; et al. Sexual Dimorphism of Metabolomic Profile in Arterial Hypertension. Sci. Rep. 2020, 10, 7517. [Google Scholar] [CrossRef] [PubMed]
- Siddik, M.A.B.; Shin, A.C. Recent Progress on Branched-Chain Amino Acids in Obesity, Diabetes, and Beyond. Endocrinol. Metab. Seoul Korea 2019, 34, 234–246. [Google Scholar] [CrossRef]
- Santoro, N.; Randolph, J.F. Reproductive Hormones and the Menopause Transition. Obstet. Gynecol. Clin. N. Am. 2011, 38, 455–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coburn, S.B.; Stanczyk, F.Z.; Falk, R.T.; McGlynn, K.A.; Brinton, L.A.; Sampson, J.; Bradwin, G.; Xu, X.; Trabert, B. Comparability of Serum, Plasma, and Urinary Estrogen and Estrogen Metabolite Measurements by Sex and Menopausal Status. Cancer Causes Control 2019, 30, 75–86. [Google Scholar] [CrossRef]
- Zlotnik, A.; Gruenbaum, B.F.; Mohar, B.; Kuts, R.; Gruenbaum, S.E.; Ohayon, S.; Boyko, M.; Klin, Y.; Sheiner, E.; Shaked, G.; et al. The Effects of Estrogen and Progesterone on Blood Glutamate Levels: Evidence from Changes of Blood Glutamate Levels during the Menstrual Cycle in Women. Biol. Reprod. 2011, 84, 581–586. [Google Scholar] [CrossRef]
- Di Stefano, G.; Di Lionardo, A.; Galosi, E.; Truini, A.; Cruccu, G. Acetyl-L-Carnitine in Painful Peripheral Neuropathy: A Systematic Review. J. Pain Res. 2019, 12, 1341–1351. [Google Scholar] [CrossRef] [Green Version]
- Scott, E.L.; Zhang, Q.-G.; Vadlamudi, R.K.; Brann, D.W. Premature Menopause and Risk of Neurological Disease: Basic Mechanisms and Clinical Implications. Mol. Cell. Endocrinol. 2014, 389, 2–6. [Google Scholar] [CrossRef] [Green Version]
- Brinton, R.D.; Yao, J.; Yin, F.; Mack, W.J.; Cadenas, E. Perimenopause as a Neurological Transition State. Nat. Rev. Endocrinol. 2015, 11, 393–405. [Google Scholar] [CrossRef]
- Alves, A.; Bassot, A.; Bulteau, A.-L.; Pirola, L.; Morio, B. Glycine Metabolism and Its Alterations in Obesity and Metabolic Diseases. Nutrients 2019, 11, 1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resseguie, M.; Song, J.; Niculescu, M.D.; da Costa, K.-A.; Randall, T.A.; Zeisel, S.H. Phosphatidylethanolamine N-Methyltransferase (PEMT) Gene Expression Is Induced by Estrogen in Human and Mouse Primary Hepatocytes. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2007, 21, 2622–2632. [Google Scholar] [CrossRef] [Green Version]
- Fischer, L.M.; da Costa, K.-A.; Kwock, L.; Galanko, J.; Zeisel, S.H. Dietary Choline Requirements of Women: Effects of Estrogen and Genetic Variation. Am. J. Clin. Nutr. 2010, 92, 1113–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G. Important Roles of Dietary Taurine, Creatine, Carnosine, Anserine and 4-Hydroxyproline in Human Nutrition and Health. Amino Acids 2020, 52, 329–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallister, T.; Jennings, A.; Mohney, R.P.; Yarand, D.; Mangino, M.; Cassidy, A.; MacGregor, A.; Spector, T.D.; Menni, C. Characterizing Blood Metabolomics Profiles Associated with Self-Reported Food Intakes in Female Twins. PLoS ONE 2016, 11, e0158568. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, J.; Wu, G. Glycine Metabolism in Animals and Humans: Implications for Nutrition and Health. Amino Acids 2013, 45, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Wittenbecher, C.; Mühlenbruch, K.; Kröger, J.; Jacobs, S.; Kuxhaus, O.; Floegel, A.; Fritsche, A.; Pischon, T.; Prehn, C.; Adamski, J.; et al. Amino Acids, Lipid Metabolites, and Ferritin as Potential Mediators Linking Red Meat Consumption to Type 2 Diabetes. Am. J. Clin. Nutr. 2015, 101, 1241–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeisel, S.H.; Mar, M.-H.; Howe, J.C.; Holden, J.M. Concentrations of Choline-Containing Compounds and Betaine in Common Foods. J. Nutr. 2003, 133, 1302–1307. [Google Scholar] [CrossRef]
- Floegel, A.; von Ruesten, A.; Drogan, D.; Schulze, M.B.; Prehn, C.; Adamski, J.; Pischon, T.; Boeing, H. Variation of Serum Metabolites Related to Habitual Diet: A Targeted Metabolomic Approach in EPIC-Potsdam. Eur. J. Clin. Nutr. 2013, 67, 1100–1108. [Google Scholar] [CrossRef]
- Altmaier, E.; Kastenmüller, G.; Römisch-Margl, W.; Thorand, B.; Weinberger, K.M.; Illig, T.; Adamski, J.; Döring, A.; Suhre, K. Questionnaire-Based Self-Reported Nutrition Habits Associate with Serum Metabolism as Revealed by Quantitative Targeted Metabolomics. Eur. J. Epidemiol. 2011, 26, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Charles, D.; Gethings, L.A.; Potts, J.F.; Burney, P.G.J.; Garcia-Larsen, V. Mass Spectrometry-Based Metabolomics for the Discovery of Candidate Markers of Flavonoid and Polyphenolic Intake in Adults. Sci. Rep. 2021, 11, 5801. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Larsen, V.; Luczynska, M.; Kowalski, M.L.; Voutilainen, H.; Ahlström, M.; Haahtela, T.; Toskala, E.; Bockelbrink, A.; Lee, H.-H.; Vassilopoulou, E.; et al. Use of a Common Food Frequency Questionnaire (FFQ) to Assess Dietary Patterns and Their Relation to Allergy and Asthma in Europe: Pilot Study of the GA2LEN FFQ. Eur. J. Clin. Nutr. 2011, 65, 750–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food Standard Agency. Food Portion Sizes Guidelines, 3rd ed.; Food Standard Agency: London, UK, 2006.
- McCane, W.; Widdowson, Y.M. The Composition of Foods Integrated Dataset; Food Standards Agency: London, UK, 2019.
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for Total Energy Intake in Epidemiologic Studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef] [PubMed]





| Metabolomics Data | Metabolomics and Diet Data | |||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| n | 3125 | 3747 | 1095 | 1231 |
| Age [mean (SD)] | 46.4 (16.5) | 45.7 (16.5) | 46.4 (16.7) | 45.0 (16.6) |
| Not fasting, n (%) | 222 (7.1%) | 233 (7.4%) | 98 (8.9%) | 70 (5.7%) |
| BMI, n (%) | ||||
| 1: underweight | 13 (0.42%) | 86 (2.3%) | 5 (0.46%) | 32 (2.6%) |
| 2: normal | 1202 (38.5%) | 2072 (55.3%) | 421 (38.5%) | 660 (53.6%) |
| 3: overweight | 1374 (43.9%) | 977 (26.0%) | 487 (44.5%) | 335 (27.2%) |
| 4: obese | 503 (16.1%) | 584 (15.6%) | 180 (16.4%) | 202 (16.4%) |
| missing | 33 | 28 | 2 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verri Hernandes, V.; Dordevic, N.; Hantikainen, E.M.; Sigurdsson, B.B.; Smárason, S.V.; Garcia-Larsen, V.; Gögele, M.; Caprioli, G.; Bozzolan, I.; Pramstaller, P.P.; et al. Age, Sex, Body Mass Index, Diet and Menopause Related Metabolites in a Large Homogeneous Alpine Cohort. Metabolites 2022, 12, 205. https://doi.org/10.3390/metabo12030205
Verri Hernandes V, Dordevic N, Hantikainen EM, Sigurdsson BB, Smárason SV, Garcia-Larsen V, Gögele M, Caprioli G, Bozzolan I, Pramstaller PP, et al. Age, Sex, Body Mass Index, Diet and Menopause Related Metabolites in a Large Homogeneous Alpine Cohort. Metabolites. 2022; 12(3):205. https://doi.org/10.3390/metabo12030205
Chicago/Turabian StyleVerri Hernandes, Vinicius, Nikola Dordevic, Essi Marjatta Hantikainen, Baldur Bragi Sigurdsson, Sigurður Vidir Smárason, Vanessa Garcia-Larsen, Martin Gögele, Giulia Caprioli, Ilaria Bozzolan, Peter P. Pramstaller, and et al. 2022. "Age, Sex, Body Mass Index, Diet and Menopause Related Metabolites in a Large Homogeneous Alpine Cohort" Metabolites 12, no. 3: 205. https://doi.org/10.3390/metabo12030205
APA StyleVerri Hernandes, V., Dordevic, N., Hantikainen, E. M., Sigurdsson, B. B., Smárason, S. V., Garcia-Larsen, V., Gögele, M., Caprioli, G., Bozzolan, I., Pramstaller, P. P., & Rainer, J. (2022). Age, Sex, Body Mass Index, Diet and Menopause Related Metabolites in a Large Homogeneous Alpine Cohort. Metabolites, 12(3), 205. https://doi.org/10.3390/metabo12030205