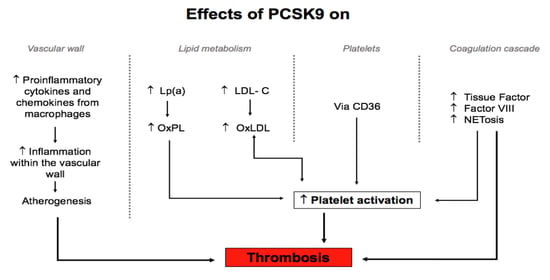
Pleiotropic Effects of PCSK9: Focus on Thrombosis and Haemostasis
Abstract
:1. Introduction
2. Effect of PCSK9 on the Vascular Wall in Relation to Atherothrombosis
3. Overview of the Effect of Lipoproteins on Platelets and Coagulation
4. Impact of PCSK9 on Platelets and Blood Coagulation
4.1. Effect of PCSK9 on Platelet Structure and Function
4.2. Effect of PCSK9 on Blood Coagulation Factors
5. The Effect of PCSK9 Inhibitors on Platelet Function and Thrombotic Risk
6. Effect of Other Lipid-Lowering Therapies on Thrombotic Risk
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Urban, D.; Poss, J.; Bohm, M.; Laufs, U. Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis. J. Am. Coll. Cardiol. 2013, 62, 1401–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Yan, B.; Gui, Y.; Tang, Z.; Tai, S.; Zhou, S.; Zheng, X.L. Physiology and role of PCSK9 in vascular disease: Potential impact of localized PCSK9 in vascular wall. J. Cell. Physiol. 2021, 236, 2333–2351. [Google Scholar] [CrossRef] [PubMed]
- Basiak, M.; Kosowski, M.; Cyrnek, M.; Buldak, L.; Maliglowka, M.; Machnik, G.; Okopien, B. Pleiotropic Effects of PCSK-9 Inhibitors. Int. J. Mol. Sci. 2021, 22, 3144. [Google Scholar] [CrossRef] [PubMed]
- Barale, C.; Melchionda, E.; Morotti, A.; Russo, I. PCSK9 Biology and Its Role in Atherothrombosis. Int. J. Mol. Sci. 2021, 22, 5880. [Google Scholar] [CrossRef]
- Gurbel, P.A.; Navarese, E.P.; Tantry, U.S. Exploration of PCSK9 as a Cardiovascular Risk Factor: Is There a Link to the Platelet? J. Am. Coll. Cardiol. 2017, 70, 1463–1466. [Google Scholar] [CrossRef]
- Ragusa, R.; Basta, G.; Neglia, D.; De Caterina, R.; Del Turco, S.; Caselli, C. PCSK9 and atherosclerosis: Looking beyond LDL regulation. Eur. J. Clin. Investig. 2021, 51, e13459. [Google Scholar] [CrossRef]
- Abifadel, M.; Varret, M.; Rabes, J.P.; Allard, D.; Ouguerram, K.; Devillers, M.; Cruaud, C.; Benjannet, S.; Wickham, L.; Erlich, D.; et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 2003, 34, 154–156. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; Fazio, S.; Giugliano, R.P.; Stroes, E.S.G.; Kanevsky, E.; Gouni-Berthold, I.; Im, K.; Lira Pineda, A.; Wasserman, S.M.; Ceska, R.; et al. Lipoprotein(a), PCSK9 Inhibition, and Cardiovascular Risk. Circulation 2019, 139, 1483–1492. [Google Scholar] [CrossRef]
- Guo, Y.; Yan, B.; Tai, S.; Zhou, S.; Zheng, X.L. PCSK9: Associated with cardiac diseases and their risk factors? Arch. Biochem. Biophys. 2021, 704, 108717. [Google Scholar] [CrossRef]
- Krahel, J.A.; Baran, A.; Kaminski, T.W.; Flisiak, I. Proprotein Convertase Subtilisin/Kexin Type 9, Angiopoietin-Like Protein 8, Sortilin, and Cholesteryl Ester Transfer Protein-Friends of Foes for Psoriatic Patients at the Risk of Developing Cardiometabolic Syndrome? Int. J. Mol. Sci. 2020, 21, 3682. [Google Scholar] [CrossRef]
- Luquero, A.; Badimon, L.; Borrell-Pages, M. PCSK9 Functions in Atherosclerosis Are Not Limited to Plasmatic LDL-Cholesterol Regulation. Front. Cardiovasc. Med. 2021, 8, 639727. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.D.; Peng, Z.S.; Gu, H.M.; Wang, M.; Wang, G.Q.; Zhang, D.W. Regulation of PCSK9 Expression and Function: Mechanisms and Therapeutic Implications. Front. Cardiovasc. Med. 2021, 8, 764038. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.H.; Li, T.H.; Peng, J.; Zheng, J.; Li, T.T.; Liu, L.S.; Jiang, Z.S.; Zheng, X.L. PCSK9: A novel inflammation modulator in atherosclerosis? J. Cell. Physiol. 2019, 234, 2345–2355. [Google Scholar] [CrossRef] [PubMed]
- Sundararaman, S.S.; Doring, Y.; van der Vorst, E.P.C. PCSK9: A Multi-Faceted Protein That Is Involved in Cardiovascular Biology. Biomedicines 2021, 9, 793. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzo, G.; Gentile, M.; Bresciani, A.; Mallardo, V.; Di Lorenzo, A.; Merone, P.; Cuomo, G.; Pacileo, M.; Sarullo, F.M.; Venturini, E.; et al. Inhibitors of Protein Convertase Subtilisin/Kexin 9 (PCSK9) and Acute Coronary Syndrome (ACS): The State-of-the-Art. J. Clin. Med. 2021, 10, 1510. [Google Scholar] [CrossRef]
- Grover, S.P.; Mackman, N. Tissue factor in atherosclerosis and atherothrombosis. Atherosclerosis 2020, 307, 80–86. [Google Scholar] [CrossRef]
- Leistner, D.M.; Krankel, N.; Meteva, D.; Abdelwahed, Y.S.; Seppelt, C.; Stahli, B.E.; Rai, H.; Skurk, C.; Lauten, A.; Mochmann, H.C.; et al. Differential immunological signature at the culprit site distinguishes acute coronary syndrome with intact from acute coronary syndrome with ruptured fibrous cap: Results from the prospective translational OPTICO-ACS study. Eur. Heart J. 2020, 41, 3549–3560. [Google Scholar] [CrossRef]
- Rauch, U.; Osende, J.I.; Fuster, V.; Badimon, J.J.; Fayad, Z.; Chesebro, J.H. Thrombus formation on atherosclerotic plaques: Pathogenesis and clinical consequences. Ann. Intern. Med. 2001, 134, 224–238. [Google Scholar] [CrossRef]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Libby, P. Inflammation in atherosclerosis. Arter. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef] [Green Version]
- Davi, G.; Patrono, C. Platelet activation and atherothrombosis. N. Engl. J. Med. 2007, 357, 2482–2494. [Google Scholar] [CrossRef] [PubMed]
- Momtazi-Borojeni, A.A.; Sabouri-Rad, S.; Gotto, A.M.; Pirro, M.; Banach, M.; Awan, Z.; Barreto, G.E.; Sahebkar, A. PCSK9 and inflammation: A review of experimental and clinical evidence. Eur. Heart J. Cardiovasc. Pharmacother. 2019, 5, 237–245. [Google Scholar] [CrossRef]
- Ricci, C.; Ruscica, M.; Camera, M.; Rossetti, L.; Macchi, C.; Colciago, A.; Zanotti, I.; Lupo, M.G.; Adorni, M.P.; Cicero, A.F.G.; et al. PCSK9 induces a pro-inflammatory response in macrophages. Sci. Rep. 2018, 8, 2267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferri, N.; Tibolla, G.; Pirillo, A.; Cipollone, F.; Mezzetti, A.; Pacia, S.; Corsini, A.; Catapano, A.L. Proprotein convertase subtilisin kexin type 9 (PCSK9) secreted by cultured smooth muscle cells reduces macrophages LDLR levels. Atherosclerosis 2012, 220, 381–386. [Google Scholar] [CrossRef]
- Giunzioni, I.; Tavori, H.; Covarrubias, R.; Major, A.S.; Ding, L.; Zhang, Y.; DeVay, R.M.; Hong, L.; Fan, D.; Predazzi, I.M.; et al. Local effects of human PCSK9 on the atherosclerotic lesion. J. Pathol. 2016, 238, 52–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mineo, C. Lipoprotein receptor signalling in atherosclerosis. Cardiovasc. Res. 2020, 116, 1254–1274. [Google Scholar] [CrossRef]
- Overton, C.D.; Yancey, P.G.; Major, A.S.; Linton, M.F.; Fazio, S. Deletion of macrophage LDL receptor-related protein increases atherogenesis in the mouse. Circ. Res. 2007, 100, 670–677. [Google Scholar] [CrossRef]
- Canuel, M.; Sun, X.; Asselin, M.C.; Paramithiotis, E.; Prat, A.; Seidah, N.G. Proprotein convertase subtilisin/kexin type 9 (PCSK9) can mediate degradation of the low density lipoprotein receptor-related protein 1 (LRP-1). PLoS ONE 2013, 8, e64145. [Google Scholar] [CrossRef]
- Schulz, R.; Schluter, K.D. PCSK9 targets important for lipid metabolism. Clin. Res. Cardiol. Suppl. 2017, 12, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Pothineni, N.V.K.; Goel, A.; Luscher, T.F.; Mehta, J.L. PCSK9 and inflammation: Role of shear stress, pro-inflammatory cytokines, and LOX-1. Cardiovasc. Res. 2020, 116, 908–915. [Google Scholar] [CrossRef]
- Tang, Z.H.; Peng, J.; Ren, Z.; Yang, J.; Li, T.T.; Li, T.H.; Wang, Z.; Wei, D.H.; Liu, L.S.; Zheng, X.L.; et al. New role of PCSK9 in atherosclerotic inflammation promotion involving the TLR4/NF-kappaB pathway. Atherosclerosis 2017, 262, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Deng, X.; Zhang, P.; Wang, X.; Fan, Y.; Zhou, S.; Mu, S.; Mehta, J.L.; Ding, Z. Blood flow patterns regulate PCSK9 secretion via MyD88-mediated pro-inflammatory cytokines. Cardiovasc. Res. 2020, 116, 1721–1732. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Liu, S.; Wang, X.; Deng, X.; Fan, Y.; Sun, C.; Wang, Y.; Mehta, J.L. Hemodynamic shear stress via ROS modulates PCSK9 expression in human vascular endothelial and smooth muscle cells and along the mouse aorta. Antioxid. Redox Signal. 2015, 22, 760–771. [Google Scholar] [CrossRef] [Green Version]
- Hoogeveen, R.M.; Opstal, T.S.J.; Kaiser, Y.; Stiekema, L.C.A.; Kroon, J.; Knol, R.J.J.; Bax, W.A.; Verberne, H.J.; Cornel, J.H.; Stroes, E.S.G. PCSK9 Antibody Alirocumab Attenuates Arterial Wall Inflammation Without Changes in Circulating Inflammatory Markers. JACC Cardiovasc. Imaging 2019, 12, 2571–2573. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.H.; Fernandez, J.A.; Deguchi, H. Plasma lipoproteins, hemostasis and thrombosis. Thromb. Haemost. 2001, 86, 386–394. [Google Scholar] [PubMed]
- Icli, A.; Aksoy, F.; Nar, G.; Kaymaz, H.; Alpay, M.F.; Nar, R.; Guclu, A.; Arslan, A.; Dogan, A. Increased Mean Platelet Volume in Familial Hypercholesterolemia. Angiology 2016, 67, 146–150. [Google Scholar] [CrossRef]
- Delluc, A.; Malecot, J.M.; Kerspern, H.; Nowak, E.; Carre, J.L.; Mottier, D.; Le Gal, G.; Lacut, K. Lipid parameters, lipid lowering drugs and the risk of venous thromboembolism. Atherosclerosis 2012, 220, 184–188. [Google Scholar] [CrossRef]
- Dentali, F.; Gessi, V.; Marcucci, R.; Gianni, M.; Grandi, A.M.; Franchini, M. Lipoprotein(a) as a Risk Factor for Venous Thromboembolism: A Systematic Review and Meta-analysis of the Literature. Semin. Thromb. Hemost. 2017, 43, 614–620. [Google Scholar] [CrossRef]
- Ashrani, A.A.; Barsoum, M.K.; Crusan, D.J.; Petterson, T.M.; Bailey, K.R.; Heit, J.A. Is lipid lowering therapy an independent risk factor for venous thromboembolism? A population-based case-control study. Thromb. Res. 2015, 135, 1110–1116. [Google Scholar] [CrossRef] [Green Version]
- Kunutsor, S.K.; Seidu, S.; Khunti, K. Statins and primary prevention of venous thromboembolism: A systematic review and meta-analysis. Lancet Haematol. 2017, 4, e83–e93. [Google Scholar] [CrossRef] [Green Version]
- Ramcharan, A.S.; Van Stralen, K.J.; Snoep, J.D.; Mantel-Teeuwisse, A.K.; Rosendaal, F.R.; Doggen, C.J. HMG-CoA reductase inhibitors, other lipid-lowering medication, antiplatelet therapy, and the risk of venous thrombosis. J. Thromb. Haemost. 2009, 7, 514–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obermayer, G.; Afonyushkin, T.; Binder, C.J. Oxidized low-density lipoprotein in inflammation-driven thrombosis. J. Thromb. Haemost. 2018, 16, 418–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podrez, E.A.; Byzova, T.V.; Febbraio, M.; Salomon, R.G.; Ma, Y.; Valiyaveettil, M.; Poliakov, E.; Sun, M.; Finton, P.J.; Curtis, B.R.; et al. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat. Med. 2007, 13, 1086–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Kakutani, M.; Naruko, T.; Ueda, M.; Narumiya, S.; Masaki, T.; Sawamura, T. Activation-dependent surface expression of LOX-1 in human platelets. Biochem. Biophys. Res. Commun. 2001, 282, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Bartimoccia, S.; Nocella, C.; Di Santo, S.; Loffredo, L.; Illuminati, G.; Lombardi, E.; Boz, V.; Del Ben, M.; De Marco, L.; et al. LDL oxidation by platelets propagates platelet activation via an oxidative stress-mediated mechanism. Atherosclerosis 2014, 237, 108–116. [Google Scholar] [CrossRef]
- Magwenzi, S.; Woodward, C.; Wraith, K.S.; Aburima, A.; Raslan, Z.; Jones, H.; McNeil, C.; Wheatcroft, S.; Yuldasheva, N.; Febbriao, M.; et al. Oxidized LDL activates blood platelets through CD36/NOX2-mediated inhibition of the cGMP/protein kinase G signaling cascade. Blood 2015, 125, 2693–2703. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.; Xin, L.; Panigrahi, S.; Zimman, A.; Wang, H.; Yakubenko, V.P.; Byzova, T.V.; Salomon, R.G.; Podrez, E.A. Novel phosphatidylethanolamine derivatives accumulate in circulation in hyperlipidemic ApoE−/− mice and activate platelets via TLR2. Blood 2016, 127, 2618–2629. [Google Scholar] [CrossRef] [Green Version]
- Hartwich, J.; Dembinska-Kiec, A.; Gruca, A.; Motyka, M.; Partyka, L.; Skrzeczynska, J.; Bzowska, M.; Pryjma, J.; Huber, J.; Leitinger, N.; et al. Regulation of platelet adhesion by oxidized lipoproteins and oxidized phospholipids. Platelets 2002, 13, 141–151. [Google Scholar] [CrossRef]
- Riches, K.; Porter, K.E. Lipoprotein(a): Cellular Effects and Molecular Mechanisms. Cholesterol 2012, 2012, 923289. [Google Scholar] [CrossRef] [Green Version]
- Discepolo, W.; Wun, T.; Berglund, L. Lipoprotein(a) and thrombocytes: Potential mechanisms underlying cardiovascular risk. Pathophysiol. Haemost. Thromb. 2006, 35, 314–321. [Google Scholar] [CrossRef]
- Owens, A.P., 3rd; Passam, F.H.; Antoniak, S.; Marshall, S.M.; McDaniel, A.L.; Rudel, L.; Williams, J.C.; Hubbard, B.K.; Dutton, J.A.; Wang, J.; et al. Monocyte tissue factor-dependent activation of coagulation in hypercholesterolemic mice and monkeys is inhibited by simvastatin. J. Clin. Investig. 2012, 122, 558–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zouaoui Boudjeltia, K.; Daher, J.; Van Antwerpen, P.; Moguilevsky, N.; Delree, P.; Ducobu, J.; Raes, M.; Badran, B.; Vanhaeverbeek, M.; Brohee, D.; et al. Exposure of endothelial cells to physiological levels of myeloperoxidase-modified LDL delays pericellular fibrinolysis. PLoS ONE 2012, 7, e38810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Stoep, M.; Korporaal, S.J.; Van Eck, M. High-density lipoprotein as a modulator of platelet and coagulation responses. Cardiovasc. Res. 2014, 103, 362–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nofer, J.R.; Brodde, M.F.; Kehrel, B.E. High-density lipoproteins, platelets and the pathogenesis of atherosclerosis. Clin. Exp. Pharmacol. Physiol. 2010, 37, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Nofer, J.R.; van Eck, M. HDL scavenger receptor class B type I and platelet function. Curr. Opin. Lipidol. 2011, 22, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ashraf, M.Z.; Podrez, E.A. Scavenger receptor BI modulates platelet reactivity and thrombosis in dyslipidemia. Blood 2010, 116, 1932–1941. [Google Scholar] [CrossRef] [Green Version]
- Kaba, N.K.; Francis, C.W.; Moss, A.J.; Zareba, W.; Oakes, D.; Knox, K.L.; Fernandez, I.D.; Rainwater, D.L.; Investigators, T. Effects of lipids and lipid-lowering therapy on hemostatic factors in patients with myocardial infarction. J. Thromb. Haemost. 2004, 2, 718–725. [Google Scholar] [CrossRef]
- Camera, M.; Rossetti, L.; Barbieri, S.S.; Zanotti, I.; Canciani, B.; Trabattoni, D.; Ruscica, M.; Tremoli, E.; Ferri, N. PCSK9 as a Positive Modulator of Platelet Activation. J. Am. Coll. Cardiol. 2018, 71, 952–954. [Google Scholar] [CrossRef]
- Navarese, E.P.; Kolodziejczak, M.; Winter, M.P.; Alimohammadi, A.; Lang, I.M.; Buffon, A.; Lip, G.Y.; Siller-Matula, J.M. Association of PCSK9 with platelet reactivity in patients with acute coronary syndrome treated with prasugrel or ticagrelor: The PCSK9-REACT study. Int. J. Cardiol. 2017, 227, 644–649. [Google Scholar] [CrossRef]
- Pastori, D.; Nocella, C.; Farcomeni, A.; Bartimoccia, S.; Santulli, M.; Vasaturo, F.; Carnevale, R.; Menichelli, D.; Violi, F.; Pignatelli, P.; et al. Relationship of PCSK9 and Urinary Thromboxane Excretion to Cardiovascular Events in Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2017, 70, 1455–1462. [Google Scholar] [CrossRef]
- Qi, Z.; Hu, L.; Zhang, J.; Yang, W.; Liu, X.; Jia, D.; Yao, Z.; Chang, L.; Pan, G.; Zhong, H.; et al. PCSK9 (Proprotein Convertase Subtilisin/Kexin 9) Enhances Platelet Activation, Thrombosis, and Myocardial Infarct Expansion by Binding to Platelet CD36. Circulation 2021, 143, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Cammisotto, V.; Pastori, D.; Nocella, C.; Bartimoccia, S.; Castellani, V.; Marchese, C.; Scavalli, A.S.; Ettorre, E.; Viceconte, N.; Violi, F.; et al. PCSK9 Regulates Nox2-Mediated Platelet Activation via CD36 Receptor in Patients with Atrial Fibrillation. Antioxidants 2020, 9, 296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barale, C.; Bonomo, K.; Frascaroli, C.; Morotti, A.; Guerrasio, A.; Cavalot, F.; Russo, I. Platelet function and activation markers in primary hypercholesterolemia treated with anti-PCSK9 monoclonal antibody: A 12-month follow-up. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, C.G.; Guo, Y.L.; Xu, R.X.; Zhang, Y.; Sun, J.; Li, J.J. The relationship between the plasma PCSK9 levels and platelet indices in patients with stable coronary artery disease. J. Atheroscler. Thromb. 2015, 22, 76–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen-Uribe, A.; Kremser, M.; Rohlfing, A.K.; Castor, T.; Kolb, K.; Dicenta, V.; Emschermann, F.; Li, B.; Borst, O.; Rath, D.; et al. Platelet-Derived PCSK9 Is Associated with LDL Metabolism and Modulates Atherothrombotic Mechanisms in Coronary Artery Disease. Int. J. Mol. Sci. 2021, 22, 11179. [Google Scholar] [CrossRef]
- Pignatelli, P.; Carnevale, R.; Di Santo, S.; Bartimoccia, S.; Sanguigni, V.; Lenti, L.; Finocchi, A.; Mendolicchio, L.; Soresina, A.R.; Plebani, A.; et al. Inherited human gp91phox deficiency is associated with impaired isoprostane formation and platelet dysfunction. Arter. Thromb. Vasc. Biol. 2011, 31, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.Q.; Qin, J.; Plow, E.F. Platelet integrin alpha(IIb)beta(3): Activation mechanisms. J. Thromb. Haemost. 2007, 5, 1345–1352. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Q.; Wang, J.; Guo, C.; Kleiman, K.; Meng, H.; Knight, J.S.; Eitzman, D.T. Proprotein convertase subtilisin/kexin type 9 (PCSK9) Deficiency is Protective Against Venous Thrombosis in Mice. Sci. Rep. 2017, 7, 14360. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Batra, S.; Jeyaseelan, S. Diminished neutrophil extracellular trap (NET) formation is a novel innate immune deficiency induced by acute ethanol exposure in polymicrobial sepsis, which can be rescued by CXCL1. PLoS Pathog. 2017, 13, e1006637. [Google Scholar] [CrossRef] [Green Version]
- Schuster, S.; Rubil, S.; Endres, M.; Princen, H.M.G.; Boeckel, J.N.; Winter, K.; Werner, C.; Laufs, U. Anti-PCSK9 antibodies inhibit pro-atherogenic mechanisms in APOE*3Leiden.CETP mice. Sci. Rep. 2019, 9, 11079. [Google Scholar] [CrossRef]
- Kimball, A.S.; Obi, A.T.; Diaz, J.A.; Henke, P.K. The Emerging Role of NETs in Venous Thrombosis and Immunothrombosis. Front. Immunol. 2016, 7, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwivedi, D.J.; Grin, P.M.; Khan, M.; Prat, A.; Zhou, J.; Fox-Robichaud, A.E.; Seidah, N.G.; Liaw, P.C. Differential Expression of PCSK9 Modulates Infection, Inflammation, and Coagulation in a Murine Model of Sepsis. Shock 2016, 46, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.A.; Oleaga, C.; Eren, M.; Amaral, A.P.; Shang, M.; Lux, E.; Khan, S.S.; Shah, S.J.; Omura, Y.; Pamir, N.; et al. Role of PAI-1 in hepatic steatosis and dyslipidemia. Sci. Rep. 2021, 11, 430. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, E.; Iriondo, M.; Manzano, C.; Fullaondo, A.; Villar, I.; Ruiz-Irastorza, G.; Zubiaga, A.M.; Estonba, A. LDLR and PCSK9 Are Associated with the Presence of Antiphospholipid Antibodies and the Development of Thrombosis in aPLA Carriers. PLoS ONE 2016, 11, e0146990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhu, C.G.; Xu, R.X.; Li, S.; Guo, Y.L.; Sun, J.; Li, J.J. Relation of circulating PCSK9 concentration to fibrinogen in patients with stable coronary artery disease. J. Clin. Lipidol. 2014, 8, 494–500. [Google Scholar] [CrossRef]
- Scalise, V.; Sanguinetti, C.; Neri, T.; Cianchetti, S.; Lai, M.; Carnicelli, V.; Celi, A.; Pedrinelli, R. PCSK9 Induces Tissue Factor Expression by Activation of TLR4/NFkB Signaling. Int. J. Mol. Sci. 2021, 22, 12640. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Liu, M.M.; Liu, H.H.; Guo, Y.L.; Wu, N.Q.; Dong, Q.; Qian, J.; Dou, K.F.; Zhu, C.G.; Li, J.J. Association of circulating proprotein convertase subtilisin/kexin type 9 concentration, prothrombin time and cardiovascular outcomes: A prospective cohort study. Thromb. J. 2021, 19, 90. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.F.; Guo, Y.G.; Chen, M.M.; Jiang, Z.L.; Song, J.Y. Positive correlation between plasma PCSK9 and tissue factors levels in patients with angiographically diagnosed coronary artery disease and diabetes mellitus. J. Geriatr. Cardiol. 2016, 13, 312–315. [Google Scholar] [CrossRef]
- Grover, S.P.; Mackman, N. Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arter. Thromb. Vasc. Biol. 2018, 38, 709–725. [Google Scholar] [CrossRef] [Green Version]
- Tatsumi, K.; Mackman, N. Tissue Factor and Atherothrombosis. J. Atheroscler. Thromb. 2015, 22, 543–549. [Google Scholar] [CrossRef] [Green Version]
- Witkowski, M.; Landmesser, U.; Rauch, U. Tissue factor as a link between inflammation and coagulation. Trends Cardiovasc. Med. 2016, 26, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Hamik, A.; Setiadi, H.; Bu, G.; McEver, R.P.; Morrissey, J.H. Down-regulation of monocyte tissue factor mediated by tissue factor pathway inhibitor and the low density lipoprotein receptor-related protein. J. Biol. Chem. 1999, 274, 4962–4969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strickland, D.K.; Au, D.T.; Cunfer, P.; Muratoglu, S.C. Low-density lipoprotein receptor-related protein-1: Role in the regulation of vascular integrity. Arter. Thromb. Vasc. Biol. 2014, 34, 487–498. [Google Scholar] [CrossRef] [Green Version]
- Qi, Z.; Sun, A.; Ge, J. Letter by Qi et al. Regarding Article, “The Effect of PCSK9 (Proprotein Convertase Subtilisin/Kexin Type 9) Inhibition on the Risk of Venous Thromboembolism”. Circulation 2020, 142, e262–e263. [Google Scholar] [CrossRef]
- Jenkins, P.V.; Rawley, O.; Smith, O.P.; O’Donnell, J.S. Elevated factor VIII levels and risk of venous thrombosis. Br. J. Haematol. 2012, 157, 653–663. [Google Scholar] [CrossRef]
- Rietveld, I.M.; Lijfering, W.M.; le Cessie, S.; Bos, M.H.A.; Rosendaal, F.R.; Reitsma, P.H.; Cannegieter, S.C. High levels of coagulation factors and venous thrombosis risk: Strongest association for factor VIII and von Willebrand factor. J. Thromb. Haemost. 2019, 17, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Saenko, E.L.; Yakhyaev, A.V.; Mikhailenko, I.; Strickland, D.K.; Sarafanov, A.G. Role of the low density lipoprotein-related protein receptor in mediation of factor VIII catabolism. J. Biol. Chem. 1999, 274, 37685–37692. [Google Scholar] [CrossRef] [Green Version]
- Bovenschen, N.; Mertens, K.; Hu, L.; Havekes, L.M.; van Vlijmen, B.J. LDL receptor cooperates with LDL receptor-related protein in regulating plasma levels of coagulation factor VIII in vivo. Blood 2005, 106, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Bank, I.; Libourel, E.J.; Middeldorp, S.; Hamulyak, K.; van Pampus, E.C.; Koopman, M.M.; Prins, M.H.; van der Meer, J.; Buller, H.R. Elevated levels of FVIII:C within families are associated with an increased risk for venous and arterial thrombosis. J. Thromb. Haemost. 2005, 3, 79–84. [Google Scholar] [CrossRef]
- Peczek, P.; Lesniewski, M.; Mazurek, T.; Szarpak, L.; Filipiak, K.J.; Gasecka, A. Antiplatelet Effects of PCSK9 Inhibitors in Primary Hypercholesterolemia. Life 2021, 11, 466. [Google Scholar] [CrossRef]
- Landmesser, U.; Haghikia, A.; Leiter, L.A.; Wright, R.S.; Kallend, D.; Wijngaard, P.; Stoekenbroek, R.; Kastelein, J.J.; Ray, K.K. Effect of inclisiran, the small-interfering RNA against proprotein convertase subtilisin/kexin type 9, on platelets, immune cells, and immunological biomarkers: A pre-specified analysis from ORION-1. Cardiovasc. Res. 2021, 117, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Marston, N.A.; Gurmu, Y.; Melloni, G.E.M.; Bonaca, M.; Gencer, B.; Sever, P.S.; Pedersen, T.R.; Keech, A.C.; Roselli, C.; Lubitz, S.A.; et al. The Effect of PCSK9 (Proprotein Convertase Subtilisin/Kexin Type 9) Inhibition on the Risk of Venous Thromboembolism. Circulation 2020, 141, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Katzmann, J.L.; Gouni-Berthold, I.; Laufs, U. PCSK9 Inhibition: Insights From Clinical Trials and Future Prospects. Front. Physiol. 2020, 11, 595819. [Google Scholar] [CrossRef]
- Schol-Gelok, S.; Galema-Boers, J.; van Gelder, T.; Kruip, M.; Roeters van Lennep, J.E.; Versmissen, J. No effect of PCSK9 inhibitors on D-dimer and fibrinogen levels in patients with familial hypercholesterolemia. Biomed. Pharm. 2018, 108, 1412–1414. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Liu, L.; Su, X.D.; Wang, B.B.; Fu, B.S.; Cui, J.Z.; Liu, X.Y. The effect of PCSK9 inhibitors on brain stroke prevention: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2234–2243. [Google Scholar] [CrossRef] [PubMed]
- Tran-Dinh, A.; Levoye, A.; Lambert, G.; Louedec, L.; Journe, C.; Meilhac, O.; Amarenco, P. Low levels of low-density lipoprotein-C associated with proprotein convertase subtilisin kexin 9 inhibition do not increase the risk of hemorrhagic transformation. Stroke 2014, 45, 3086–3088. [Google Scholar] [CrossRef] [Green Version]
- Lacoste, L.; Lam, J.Y.; Hung, J.; Letchacovski, G.; Solymoss, C.B.; Waters, D. Hyperlipidemia and coronary disease. Correction of the increased thrombogenic potential with cholesterol reduction. Circulation 1995, 92, 3172–3177. [Google Scholar] [CrossRef]
- Piorkowski, M.; Fischer, S.; Stellbaum, C.; Jaster, M.; Martus, P.; Morguet, A.J.; Schultheiss, H.P.; Rauch, U. Treatment with ezetimibe plus low-dose atorvastatin compared with higher-dose atorvastatin alone: Is sufficient cholesterol-lowering enough to inhibit platelets? J. Am. Coll. Cardiol. 2007, 49, 1035–1042. [Google Scholar] [CrossRef] [Green Version]
- Nenna, A.; Nappi, F.; Lusini, M.; Satriano, U.M.; Schiliro, D.; Spadaccio, C.; Chello, M. Effect of Statins on Platelet Activation and Function: From Molecular Pathways to Clinical Effects. BioMed Res. Int. 2021, 2021, 6661847. [Google Scholar] [CrossRef]
- Rauch, U.; Osende, J.I.; Chesebro, J.H.; Fuster, V.; Vorchheimer, D.A.; Harris, K.; Harris, P.; Sandler, D.A.; Fallon, J.T.; Jayaraman, S.; et al. Statins and cardiovascular diseases: The multiple effects of lipid-lowering therapy by statins. Atherosclerosis 2000, 153, 181–189. [Google Scholar] [CrossRef]
- Szczeklik, A.; Musial, J.; Undas, A.; Gajewski, P.; Gora, P.; Swadzba, J.; Jankowski, M. Inhibition of thrombin generation by simvastatin and lack of additive effects of aspirin in patients with marked hypercholesterolemia. J. Am. Coll. Cardiol. 1999, 33, 1286–1293. [Google Scholar] [CrossRef] [Green Version]
- Verdoia, M.; Pergolini, P.; Rolla, R.; Nardin, M.; Schaffer, A.; Barbieri, L.; Daffara, V.; Marino, P.; Bellomo, G.; Suryapranata, H.; et al. Impact of high-dose statins on vitamin D levels and platelet function in patients with coronary artery disease. Thromb. Res. 2017, 150, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, J.S.; Kruip, M.; van der Meer, F.J.; Rosendaal, F.R.; Leebeek, F.W.G.; Cannegieter, S.C.; Lijfering, W.M. Rosuvastatin use improves measures of coagulation in patients with venous thrombosis. Eur. Heart J. 2018, 39, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Paciullo, F.; Gresele, P. Effect of statins on measures of coagulation: Potential role of low-density lipoprotein receptors. Eur. Heart J. 2019, 40, 392. [Google Scholar] [CrossRef]
- Sahebkar, A.; Serban, C.; Ursoniu, S.; Mikhailidis, D.P.; Undas, A.; Lip, G.Y.; Bittner, V.; Ray, K.; Watts, G.F.; Hovingh, G.K.; et al. The impact of statin therapy on plasma levels of von Willebrand factor antigen. Systematic review and meta-analysis of randomised placebo-controlled trials. Thromb. Haemost. 2016, 115, 520–532. [Google Scholar] [CrossRef]
- Sanz-Cuesta, B.E.; Saver, J.L. Lipid-Lowering Therapy and Hemorrhagic Stroke Risk: Comparative Meta-Analysis of Statins and PCSK9 Inhibitors. Stroke 2021, 52, 3142–3150. [Google Scholar] [CrossRef]
- Becher, T.; Schulze, T.J.; Schmitt, M.; Trinkmann, F.; El-Battrawy, I.; Akin, I.; Kalsch, T.; Borggrefe, M.; Stach, K. Ezetimibe inhibits platelet activation and uPAR expression on endothelial cells. Int. J. Cardiol. 2017, 227, 858–862. [Google Scholar] [CrossRef]
- Hussein, O.; Minasian, L.; Itzkovich, Y.; Shestatski, K.; Solomon, L.; Zidan, J. Ezetimibe’s effect on platelet aggregation and LDL tendency to peroxidation in hypercholesterolaemia as monotherapy or in addition to simvastatin. Br. J. Clin. Pharmacol. 2008, 65, 637–645. [Google Scholar] [CrossRef] [Green Version]
- Camargo, L.M.; Franca, C.N.; Izar, M.C.; Bianco, H.T.; Lins, L.S.; Barbosa, S.P.; Pinheiro, L.F.; Fonseca, F.A. Effects of simvastatin/ezetimibe on microparticles, endothelial progenitor cells and platelet aggregation in subjects with coronary heart disease under antiplatelet therapy. Braz. J. Med. Biol. Res. 2014, 47, 432–437. [Google Scholar] [CrossRef]
- Miller, M.; DiNicolantonio, J.J.; Can, M.; Grice, R.; Damoulakis, A.; Serebruany, V.L. The effects of ezetimibe/simvastatin versus simvastatin monotherapy on platelet and inflammatory biomarkers in patients with metabolic syndrome. Cardiology 2013, 125, 74–77. [Google Scholar] [CrossRef]
- Barter, P.J.; Rye, K.A. Cholesteryl ester transfer protein inhibition as a strategy to reduce cardiovascular risk. J. Lipid Res. 2012, 53, 1755–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Li, W.; Hao, L.; Xie, H.; Hao, C.; Liu, C.; Li, W.; Xiong, X.; Zhao, D. The therapeutic potential of CETP inhibitors: A patent review. Expert Opin. Ther. Pat. 2018, 28, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Elseweidy, M.M.; Amin, R.S.; Atteia, H.H.; El-Zeiky, R.R.; Al-Gabri, N.A. New Insight on a Combination of Policosanol and 10-Dehydrogingerdione Phytochemicals as Inhibitors for Platelet Activation Biomarkers and Atherogenicity Risk in Dyslipidemic Rabbits: Role of CETP and PCSK9 Inhibition. Appl. Biochem. Biotechnol. 2018, 186, 805–815. [Google Scholar] [CrossRef] [PubMed]
- El-Seweidy, M.M.; Sarhan Amin, R.; Husseini Atteia, H.; El-Zeiky, R.R.; Al-Gabri, N.A. Dyslipidemia induced inflammatory status, platelet activation and endothelial dysfunction in rabbits: Protective role of 10-Dehydrogingerdione. Biomed. Pharm. 2019, 110, 456–464. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puccini, M.; Landmesser, U.; Rauch, U. Pleiotropic Effects of PCSK9: Focus on Thrombosis and Haemostasis. Metabolites 2022, 12, 226. https://doi.org/10.3390/metabo12030226
Puccini M, Landmesser U, Rauch U. Pleiotropic Effects of PCSK9: Focus on Thrombosis and Haemostasis. Metabolites. 2022; 12(3):226. https://doi.org/10.3390/metabo12030226
Chicago/Turabian StylePuccini, Marianna, Ulf Landmesser, and Ursula Rauch. 2022. "Pleiotropic Effects of PCSK9: Focus on Thrombosis and Haemostasis" Metabolites 12, no. 3: 226. https://doi.org/10.3390/metabo12030226
APA StylePuccini, M., Landmesser, U., & Rauch, U. (2022). Pleiotropic Effects of PCSK9: Focus on Thrombosis and Haemostasis. Metabolites, 12(3), 226. https://doi.org/10.3390/metabo12030226