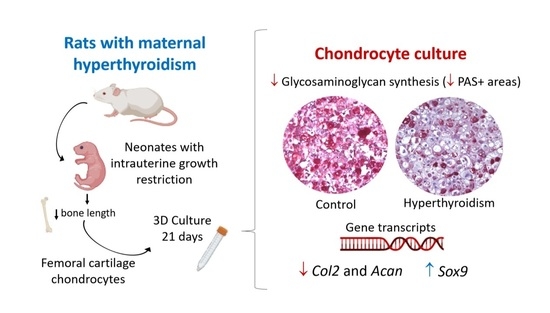
Maternal Hyperthyroidism in Rats Alters the Composition and Gene Expression of the Matrix Produced In Vitro by Chondrocytes from Offspring with Intrauterine Growth Restriction
Abstract
:1. Introduction
2. Results
2.1. Confirmation of Maternal Hyperthyroidism
2.2. Confirmation of Reduced Body Weight and Bone Length Caused by Maternal Hyperthyroidism
2.3. Maternal Hyperthyroidism Does Not Alter Viability and Alkaline-Phosphatase Activity of Offspring Chondrocytes
2.4. Maternal Hyperthyroidism Reduces Glycosaminoglycan Synthesis by Offspring Chondrocytes
2.5. Maternal Hyperthyroidism Reduces the Expression of Col 2 and Acan in Offspring Chondrocytes
3. Discussion
4. Materials and Methods
4.1. Mating and Thyroxine Administration
4.2. Dosage of Maternal Free T4 and Histomorphometric Analysis of the Neonate Thyroids
4.3. Body Weight and Femur Length Measurement
4.4. Isolation and Culture of Chondrocytes
4.5. Cell Viability Test (Conversion of MTT to Formazan Crystals)
4.6. Alkaline-Phosphatase Activity by BCIP/NBT Method
4.7. Glycosaminoglycan Analysis
4.8. Evaluation of Gene Transcript Expression
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boelaert, K.; Franklyn, J.A. Thyroid hormone in health and disease. J. Endocrinol. 2005, 187, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Krassas, G.E.; Poppe, K.; Glinoer, D. Thyroid Function and Human Reproductive Health. Endocr. Rev. 2010, 31, 702–755. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, A.H.; Czernichow, P.; Reba, R.C.; Tyson, J.; Blizzard, R.M. Observations on the maturation of thyroid function in early fetal life. J. Clin. Investig. 1970, 49, 1790–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorpe-Beeston, J.G.; Nicolaides, K.H.; Felton, C.V.; Butler, J.; McGregor, A.M. Maturation of the Secretion of Thyroid Hormone and Thyroid-Stimulating Hormone in the Fetus. N. Engl. J. Med. 1991, 324, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.L.; Andersen, S. Hyperthyroidism in pregnancy: Evidence and hypothesis in fetal programming and development. Endocr. Connect. 2021, 10, R77–R86. [Google Scholar] [CrossRef]
- Souza, C.A.; Ocarino, N.M.; Silva, J.F.; Boeloni, J.N.; Nascimento, E.F.; Silva, I.J.; Castro, R.D.; Moreira, L.P.; Almeida, F.R.C.L.; Chiarini-Garcia, H.; et al. Administration of Thyroxine Affects the Morphometric Parameters and VEGF Expression in the Uterus and Placenta and the Uterine Vascularization but does Not Affect Reproductive Parameters in Gilts During Early Gestation. Reprod. Domest. Anim. 2011, 46, e7–e16. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.F.; Ocarino, N.M.; Serakides, R. Maternal thyroid dysfunction affects placental profile of inflammatory mediators and the intrauterine trophoblast migration kinetics. Reproduction 2014, 147, 803–816. [Google Scholar] [CrossRef]
- Silva, J.F.; Ocarino, N.M.; Serakides, R. Placental angiogenic and hormonal factors are affected by thyroid hormones in rats. Pathol. Res. Pract. 2015, 211, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.A.; Silva, J.F.; Silva, C.L.R.; Ocarino, N.M.; Serakides, R. Thyroid hormones affect decidualization and angiogenesis in the decidua and metrial gland of rats. Pesqui. Vet. Bras. 2017, 37, 1002–1014. [Google Scholar] [CrossRef] [Green Version]
- Freitas, E.S.; Leite, E.D.; Souza, C.A.; Ocarino, N.M.; Ferreira, E.; Cassali, G.D.; Gomes, M.G.; Serakides, R. Histomorphometry and expression of Cdc47 and caspase-3 in hyperthyroid rat uteri and placentas during gestation and postpartum associated with fetal development. Reprod. Fertil. Dev. 2007, 19, 498–509. [Google Scholar] [CrossRef]
- Li, Q.-X.; Wang, L.-L.; Wang, Y.-Z.; Liu, L.; Han, H.; Chen, L.-B.; Wang, H. Programming changes in GLUT1 mediated the accumulation of AGEs and matrix degradation in the articular cartilage of female adult rats after prenatal caffeine exposure. Pharmacol. Res. 2020, 151, 104555. [Google Scholar] [CrossRef]
- Maia, M.Z.; Santos, G.K.; Batista, A.C.M.; Reis, A.M.S.; Silva, J.F.; Ribeiro, L.G.R.; Ocarino, N.M.; Serakides, R. Effects of excess maternal thyroxin on the bones of rat offspring from birth to the post-weaning period. Arch. Endocrinol. Metab. 2016, 60, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, L.G.R.; Silva, J.F.; Ocarino, N.M.; De Souza, C.A.; De Melo, E.G.; Serakides, R. Excess Maternal Thyroxine Alters the Proliferative Activity and Angiogenic Profile of Growth Cartilage of Rats at Birth and Weaning. Cartilage 2018, 9, 89–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassett, J.H.D.; Williams, G.R. Thyroid Hormone in Bone and Joint Disorders, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128041826. [Google Scholar]
- Williams, G.R.; Bassett, J.H.D. Thyroid diseases and bone health. J. Endocrinol. Investig. 2018, 41, 99–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozhemyakina, E.; Lassar, A.B.; Zelzer, E. A pathway to bone: Signaling molecules and transcription factors involved in chondrocyte development and maturation. Development 2015, 142, 817–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouveia, C.H.A.; Miranda-Rodrigues, M.; Martins, G.M.; Neofiti-Papi, B. Thyroid hormone and skeletal development. In Vitamins and Hormones; Elsevier: Amsterdam, The Netherlands, 2018; Volume 106, pp. 383–472. ISBN 0083-6729. [Google Scholar]
- Bassett, J.H.D.; Williams, G.R. Role of Thyroid Hormones in Skeletal Development and Bone Maintenance. Endocr. Rev. 2016, 37, 135–187. [Google Scholar] [CrossRef] [Green Version]
- Bohme, K.; Conscience-Egli, M.; Tschan, T.; Winterhalter, K.H.; Bruckner, P. Induction of proliferation or hypertrophy of chondrocytes in serum-free culture: The role of insulin-like growth factor-I, insulin, or thyroxine. J. Cell Biol. 1992, 116, 1035–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, Y.; Genge, B.R.; Wuthier, R.E.; Wu, L.N.Y. Thyroid hormone inhibits growth and stimulates terminal differentiation of epiphyseal growth plate chondrocytes. J. Bone Miner. Res. 1998, 13, 1398–1411. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Tanaka, K.; Komatsu, Y.; Suda, M.; Yasoda, A.; Sakuma, Y.; Ozasa, A.; Nakao, K. Thyroid hormones promote chondrocyte differentiation in mouse ATDC5 cells and stimulate endochondral ossification in fetal mouse tibias through iodothyronine deiodinases in the growth plate. J. Bone Miner. Res. 2002, 17, 443–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alini, M.; Kofsky, Y.; Wu, W.; Pidoux, I.; Poole, A.R. In serum-free culture thyroid hormones can induce full expression of chondrocyte hypertrophy leading to matrix calcification. J. Bone Miner. Res. 1996, 11, 105–113. [Google Scholar] [CrossRef]
- Ribeiro, L.G.R.; Silva, J.F.; Ocarino, N.M.; de Melo, E.G.; Serakides, R. Excess maternal and postnatal thyroxine alters chondrocyte numbers and the composition of the extracellular matrix of growth cartilage in rats. Connect. Tissue Res. 2018, 59, 73–84. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Abd El-Tawab, S.M.; Ahmed, R.G. Effects of experimentally induced maternal hypothyroidism and hyperthyroidism on the development of rat offspring: I. The development of the thyroid hormones-neurotransmitters and adenosinergic system interactions. Int. J. Dev. Neurosci. 2010, 28, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Kilby, M.D. Thyroid hormone and central nervous system development. J. Endocrinol. 2000, 165, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Neale, D.M.; Cootauco, A.C.; Burrow, G. Thyroid Disease in Pregnancy. Clin. Perinatol. 2007, 34, 543–557. [Google Scholar] [CrossRef]
- Vulsma, T.; Gons, M.H.; de Vijlder, J.J.M. Maternal-Fetal Transfer of Thyroxine in Congenital Hypothyroidism Due to a Total Organification Defect or Thyroid Agenesis. N. Engl. J. Med. 1989, 321, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Kiviranta, I.; Tammi, M.; Jurvelin, J.; Säämänen, A.M.; Helminen, H.J. Demonstration of chondroitin sulphate and glycoproteins in articular cartilage matrix using periodic acid-Schiff (PAS) method. Histochemistry 1985, 83, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Bassleer, C.; Gysen, P.; Foidart, J.M.; Bassleer, R.; Franchimont, P. Human chondrocytes in tridimensional culture. Vitr. Cell. Dev. Biol. 1986, 22, 113–119. [Google Scholar] [CrossRef]
- Shao, Y.Y.; Wang, L.; Ballock, R.T. Thyroid hormone and the growth plate. Rev. Endocr. Metab. Disord. 2006, 7, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.A.; Hasserjian, R.P.; Robson, H.; Siebler, T.; Shalet, S.M.; Williams, G.R. Thyroid hormones regulate hypertrophic chondrocyte differentiation and expression of parathyroid hormone-related peptide and its receptor during endochondral bone formation. J. Bone Miner. Res. 2000, 15, 2431–2442. [Google Scholar] [CrossRef] [PubMed]
- Bassett, J.H.D.; Swinhoe, R.; Chassande, O.; Samarut, J.; Williams, G.R. Thyroid hormone regulates heparan sulfate proteoglycan expression in the growth plate. Endocrinology 2006, 147, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, L.G.R. Efeito do Excesso de Tiroxina Materna e Pós-Natal Sobre o Perfil Proliferativo, Angiogênico e de Síntese das Cartilagens de Crescimento de Ratos; Universidade Federal de Minas Gerais: Belo Horizonte, Brazil, 2016. [Google Scholar]
- Williams, G.R. Thyroid Hormone Actions in Cartilage and Bone. Eur. Thyroid J. 2013, 2, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Makihira, S.; Yan, W.; Murakami, H.; Furukawa, M.; Kawai, T.; Nikawa, H.; Yoshida, E.; Hamada, T.; Okada, Y.; Kato, Y. Thyroid hormone enhances aggrecanase-2/ADAM-TS5 expression and proteoglycan degradation in growth plate cartilage. Endocrinology 2003, 144, 2480–2488. [Google Scholar] [CrossRef] [Green Version]
- Roughley, P.J. The structure and function of cartilage proteoglycans. Eur. Cells Mater. 2006, 12, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Poole, A.R.; Kobayashi, M.; Yasuda, T.; Laverty, S.; Mwale, F.; Kojima, T.; Sakai, T.; Wahl, C.; El-Maadawy, S.; Webb, G.; et al. Type II collagen degradation and its regulation in articular cartilage in osteoarthritis. Ann. Rheum. Dis. 2002, 61, 78–81. [Google Scholar] [CrossRef] [Green Version]
- Lakatos, P.A.; Bakos, B.; Takacs, I.; Stern, P.H. Thyroid hormone and bone. In Principles of Bone Biology; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 895–914. ISBN 9780128148419. [Google Scholar]
- Samsa, W.E.; Zhou, X.; Zhou, G. Signaling pathways regulating cartilage growth plate formation and activity. Semin. Cell Dev. Biol. 2017, 62, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Park, K.H. Regulation and function of SOX9 during cartilage development and regeneration. Semin. Cancer Biol. 2020, 67, 12–23. [Google Scholar] [CrossRef]
- Zwickl, H.; Niculescu-Morzsa, E.; Halbwirth, F.; Bauer, C.; Jeyakumar, V.; Reutterer, A.; Berger, M.; Nehrer, S. Correlation Analysis of SOX9, -5, and -6 as well as COL2A1 and Aggrecan Gene Expression of Collagen I Implant–Derived and Osteoarthritic Chondrocytes. Cartilage 2016, 7, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Aigner, T.; Gebhard, P.M.; Schmid, E.; Bau, B.; Harley, V.; Pöschl, E. SOX9 expression does not correlate with type II collagen expression in adult articular chondrocytes. Matrix Biol. 2003, 22, 363–372. [Google Scholar] [CrossRef]
- Lefebvre, V.; Agelozzi, M.; Haseeb, A. SOX9 in cartilage development and disease. Curr. Opin. Cell Biol. 2019, 61, 39–47. [Google Scholar] [CrossRef]
- Kim, Y.; Murao, H.; Yamamoto, K.; Deng, J.M.; Behringer, R.R.; Nakamura, T.; Akiyama, H. Generation of transgenic mice for conditional overexpression of Sox9. J. Bone Miner. Metab. 2011, 29, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Hattori, T.; Müller, C.; Gebhard, S.; Bauer, E.; Pausch, F.; Schlund, B.; Bösl, M.R.; Hess, A.; Surmann-Schmitt, C.; Von Der Mark, H.; et al. SOX9 is a major negative regulator of cartilage vascularization, bone marrow formation and endochondral ossification. Development 2010, 137, 901–911. [Google Scholar] [CrossRef] [Green Version]
- Zelzer, E.; McLean, W.; Ng, Y.S.; Fukai, N.; Reginato, A.M.; Lovejoy, S.; D’Amore, P.A.; Olsen, B.R. Skeletal defects in VEGF120/120 mice reveal multiple roles for VEGF in skeletogenesis. Development 2002, 129, 1893–1904. [Google Scholar] [CrossRef] [PubMed]
- Carlevaro, M.F.; Cermelli, S.; Cancedda, R.; Cancedda, F.D. Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: Auto-paracrine role during endochondral bone formation. J. Cell Sci. 2000, 113, 59–69. [Google Scholar] [CrossRef]
- Gerber, H.P.; Ferrara, N. Angiogenesis and bone growth. Trends Cardiovasc. Med. 2000, 10, 223–228. [Google Scholar] [CrossRef]
- Serakides, R.; Nunes, V.A.; Ocarino, N.D.M.; Nascimento, E.F.D. Efeito da Associação Hipertireoidismo- Castração no Osso de Ratas Adultas. Arq. Bras. Endocrinol. Metabol. 2004, 48, 875–884. [Google Scholar] [CrossRef] [Green Version]
- Tamiasso, N.V.; Silva, C.M.O.; Reis, A.M.S.; Ocarino, N.M.; Serakides, R. Ethanol Alters Phenotype and Synthesis Activity of Rat Neonatal Articular Chondrocytes Grown in 2- and 3-Dimensional Culture. Cartilage 2019, 13, 839S–846S. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, F.R.; Bertassoli, B.M.; Ocarino, N.M.; Reis, A.M.S.; Silva, J.F.; Ribeiro, L.G.R.; Serakides, R. Maternal Hyperthyroidism in Rats Alters the Composition and Gene Expression of the Matrix Produced In Vitro by Chondrocytes from Offspring with Intrauterine Growth Restriction. Metabolites 2022, 12, 292. https://doi.org/10.3390/metabo12040292
Araújo FR, Bertassoli BM, Ocarino NM, Reis AMS, Silva JF, Ribeiro LGR, Serakides R. Maternal Hyperthyroidism in Rats Alters the Composition and Gene Expression of the Matrix Produced In Vitro by Chondrocytes from Offspring with Intrauterine Growth Restriction. Metabolites. 2022; 12(4):292. https://doi.org/10.3390/metabo12040292
Chicago/Turabian StyleAraújo, Fabiana R., Bruno M. Bertassoli, Natália M. Ocarino, Amanda M. S. Reis, Juneo F. Silva, Lorena G. R. Ribeiro, and Rogéria Serakides. 2022. "Maternal Hyperthyroidism in Rats Alters the Composition and Gene Expression of the Matrix Produced In Vitro by Chondrocytes from Offspring with Intrauterine Growth Restriction" Metabolites 12, no. 4: 292. https://doi.org/10.3390/metabo12040292
APA StyleAraújo, F. R., Bertassoli, B. M., Ocarino, N. M., Reis, A. M. S., Silva, J. F., Ribeiro, L. G. R., & Serakides, R. (2022). Maternal Hyperthyroidism in Rats Alters the Composition and Gene Expression of the Matrix Produced In Vitro by Chondrocytes from Offspring with Intrauterine Growth Restriction. Metabolites, 12(4), 292. https://doi.org/10.3390/metabo12040292