Sex Dimorphism in the Metabolome of Metabolic Syndrome in Morbidly Obese Individuals
Abstract
:1. Introduction
2. Results
2.1. Population Characteristics and MetS Incidence
2.2. The Metabolomic Differences between MHO and MetS Are Age-Dependent
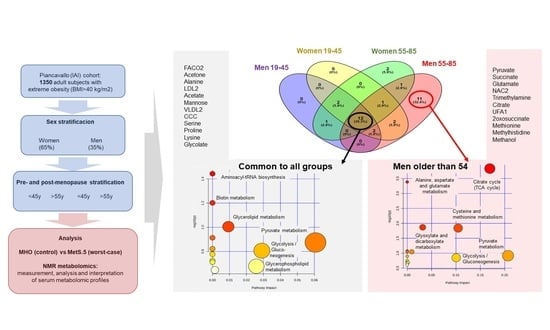
2.3. Sex Influence on MetS Metabolomic Impact in the Context of Severe Obesity
2.4. MetS Produces Metabolomic Changes in Male Older Than 55 That Are Different from Those in Other Subgroups
3. Discussion
4. Materials and Methods
4.1. Subjects and Study Design
4.2. Sample Collection and Preparation
4.3. Metabolite Quantification
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, G.A. Risks of obesity. Endocrinol. Metab. Clin. N. Am. 2003, 32, 787–804, viii. [Google Scholar] [CrossRef]
- Zembic, A.; Eckel, N.; Stefan, N.; Baudry, J.; Schulze, M.B. An Empirically Derived Definition of Metabolically Healthy Obesity Based on Risk of Cardiovascular and Total Mortality. JAMA Netw. Open 2021, 4, e218505. [Google Scholar] [CrossRef] [PubMed]
- Tsatsoulis, A.; Paschou, S.A. Metabolically Healthy Obesity: Criteria, Epidemiology, Controversies, and Consequences. Curr. Obes. Rep. 2020, 9, 109–120. [Google Scholar] [CrossRef] [PubMed]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.; Shaw, J.; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome--a new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Paragh, G.; Seres, I.; Harangi, M.; Fülöp, P. Dynamic interplay between metabolic syndrome and immunity. Adv. Exp. Med. Biol. 2014, 824, 171–190. [Google Scholar]
- Marrachelli, V.G.; Rentero, P.; Mansego, M.L.; Morales, J.M.; Galan, I.; Pardo-Tendero, M.; Martínez, F.; Martín-Escudero, J.C.; Briongos, L.; Chaves, F.J.; et al. Genomic and Metabolomic Profile Associated to Clustering of Cardio-Metabolic Risk Factors. PLoS ONE 2016, 11, e0160656. [Google Scholar] [CrossRef]
- Canale, M.P.; di Villahermosa, S.M.; Martino, G.; Rovella, V.; Noce, A.; De Lorenzo, A.; Di Daniele, N. Obesity-related metabolic syndrome: Mechanisms of sympathetic overactivity. Int. J. Endocrinol. 2013, 2013, 865965. [Google Scholar] [CrossRef] [Green Version]
- Ervin, R.B. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl. Health Stat. Rep. 2009, 5, 1–7. [Google Scholar]
- Ford, E.S.; Giles, W.H.; Dietz, W.H. Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. JAMA 2002, 287, 356–359. [Google Scholar] [CrossRef]
- Kotani, K.; Tokunaga, K.; Fujioka, S.; Kobatake, T.; Keno, Y.; Yoshida, S.; Shimomura, I.; Tarui, S.; Matsuzawa, Y. Sexual dimorphism of age-related changes in whole-body fat distribution in the obese. Int. J. Obes. Relat. Metab. Disord. 1994, 18, 202–207. [Google Scholar] [PubMed]
- Muller, M.; Grobbee, D.E.; den Tonkelaar, I.; Lamberts, S.W.; van der Schouw, Y.T. Endogenous sex hormones and metabolic syndrome in aging men. J. Clin. Endocrinol. Metab. 2005, 90, 2618–2623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuk, J.L.; Ardern, C.I. Age and sex differences in the clustering of metabolic syndrome factors: Association with mortality risk. Diabetes Care 2010, 33, 2457–2461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangel-Huerta, O.D.; Pastor-Villaescusa, B.; Gil, A. Are we close to defining a metabolomic signature of human obesity? A systematic review of metabolomics studies. Metabolomics 2019, 15, 930-y. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, P.; Bouchard, B. The Impact of Aging on Adipose Function and Adipokine Synthesis. Front. Endocrinol. 2019, 10, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pucci, G.; Alcidi, R.; Tap, L.; Battista, F.; Mattace-Raso, F.; Schillaci, G. Sex- and gender-related prevalence, cardiovascular isk and therapeutic approach in metabolic syndrome: A review of the literature. Pharmacol. Res. 2017, 120, 34–42. [Google Scholar] [CrossRef]
- Holmes, D. Biomarkers: Mannose levels predict insulin resistance. Nat. Rev. Endocrinol. 2016, 12, 496. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Monleon, D.; Verhamme, P.; Staessen, J.A. Branched-Chain Amino Acids as Critical Switches in Health and Disease. Hypertension 2018, 72, 1012–1022. [Google Scholar] [CrossRef]
- Cani, P.D.; Delzenne, N.M. Interplay between obesity and associated metabolic disorders: New insights into the gut microbiota. Curr. Opin. Pharmacol. 2009, 9, 737–743. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, A.P.; de Souza Santos, R.; Palmer, B.F.; Clegg, D.J. Determinants of body fat distribution in humans may provide insight about obesity-related health risks. J. Lipid Res. 2019, 60, 1710–1719. [Google Scholar] [CrossRef] [PubMed]
- Meeta Digumarti, L.; Agarwal, N.; Vaze, N.; Shah, R.; Malik, S. Clinical practice guidelines on menopause: An executive summary and recommendations. J. Midlife Health 2013, 4, 77–106. [Google Scholar]
- Krebs, H.A. The regulation of the release of ketone bodies by the liver. Adv. Enzyme Regul. 1966, 4, 339–354. [Google Scholar] [CrossRef]
- Borrás, C.; Sastre, J.; García-Sala, D.; Lloret, A.; Pallardó, F.V.; Viña, J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol. Med. 2003, 34, 546–552. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancello, R.; Soranna, D.; Brunani, A.; Scacchi, M.; Tagliaferri, A.; Mai, S.; Marzullo, P.; Zambon, A.; Invitti, C. Analysis of predictive equations for estimating resting energy expenditure in a large cohort of morbidly obese patients. Front. Endocrinol. 2018, 9, 367. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. Sporadic mutations in melanocortin receptor 3 in morbid obese individuals. Eur. J. Hum. Genet. 2008, 16, 581–586. [Google Scholar]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]





| Variables | All Cohort (n = 1350) | Males (n = 465) | Females (n = 885) | p-Value |
|---|---|---|---|---|
| Age (years) | 53.66 ± 13.84 | 50.34 ± 13.67 | 55.41 ± 13.61 | +++ |
| Height (cm) | 162.55 ± 10.5 | 172.96 ± 7.75 | 157.08 ± 7.06 | +++ |
| Weight (kg) | 126.54 ± 23.85 | 142.89 ± 24.07 | 117.96 ± 18.7 | +++ |
| BMI (kg m−2) | 47.7 ± 6.54 | 47.63 ± 6.56 | 47.74 ± 6.52 | |
| Waist circumference (cm) | 133.27 ± 14.61 | 142.8 ± 13.1 | 128.25 ± 12.75 | +++ |
| Hip circumference (cm) | 140.68 ± 14.45 | 139.34 ± 15.47 | 141.39 ± 13.82 | |
| WHR (cm/cm) | 0.95 ± 0.1 | 1.03 ± 0.07 | 0.91 ± 0.08 | +++ |
| Systolic blood pressure (mm Hg) | 138.68 ± 17.61 | 140.9 ± 17.46 | 137.51 ± 17.58 | |
| Diastolic blood pressure (mm Hg) | 82.56 ± 9.31 | 84.42 ± 9.07 | 81.58 ± 9.28 | +++ |
| SatO2 | 93.51 ± 2.83 | 93.24 ± 2.47 | 93.65 ± 3 | |
| HOMA-IR | 4.39 ± 3.27 | 4.88 ± 2.85 | 4.14 ± 3.43 | |
| s-Glucose (mg/dL) | 112.29 ± 35.73 | 112.89 ± 34.05 | 111.98 ± 36.57 | |
| s-LDL Cholesterol (mg/dL) | 120.19 ± 34.87 | 119.53 ± 35.93 | 120.53 ± 34.29 | |
| s-HDL Cholesterol (mg/dL) | 42.67 ± 12.4 | 36.86 ± 9.68 | 45.73 ± 12.58 | +++ |
| s-Triglycerides (mg/dL) | 144.1 ± 62.13 | 157.05 ± 60.92 | 137.3 ± 61.68 | +++ |
| s-Insuline (mU/L) | 15.8 ± 9.4 | 17.95 ± 8.96 | 14.59 ± 9.42 | +++ |
| cc_H2O tot | 39.14 ± 15.75 | 43.59 ± 4.38 | 36.81 ± 18.76 | +++ |
| cc_fat mass | 49.52 ± 19.84 | 41.52 ± 5.7 | 53.72 ± 23.05 | +++ |
| cc_free-fat mass | 51.61 ± 18.1 | 58.21 ± 6 | 48.16 ± 21.11 | +++ |
| cc_muscular mass | 29.64 ± 11.51 | 33.56 ± 6.49 | 27.59 ± 12.9 | +++ |
| Basal metabolic rate | 1995.77 ± 460.24 | 2376.72 ± 439.62 | 1777.38 ± 302.61 | +++ |
| Resting energy expenditure X day | 2074.92 ± 457.13 | 2540.59 ± 395.5 | 1807.6 ± 207.8 | +++ |
| Resting energy expenditure% | 96.55 ± 11.14 | 93.74 ± 10.69 | 98.16 ± 11.08 | +++ |
| Piancavallo Cohort Composition n (%) | Total | p-Value Age Ranges | Women | Men | p-Value Women vs. Men (by Age Ranges) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Ages 1350 | Range 19–45 363 (26.9%) | Range 55–85 723 (53.6%) | All Ages 885 (65.5%) | Range 19–45 196 (22.1%) | Range 55–85 520 (58.8%) | All Ages 465 (34.5%) | Range 19–45 167 (35.9%) | Range 55–85 203 (43.7%) | ||||
| Metabolic profile | MHO | 264 (19.5%) | 94 (25.9%) | 127 (17.6%) | 196 (22.1%) | 60 (30.6%) | 106 (20.4%) | 68 (14.6%) | 34 (20.6%) | 21 (10.3%) | ||
| MetS | 1086 (80.5%) | 269 (74.1%) | 596 (82.4%) | 689 (77.8%) | 136 (69.4%) | 414 (79.6%) | 397 (85.4%) | 133 (79.6%) | 182 (89.7%) | |||
| MetS.5 | 256 (23.6%) | 46 (17.1%) | 144 (24.2%) | 153 (22.2%) | 15 (11%) | 96 (23.2%) | 103 (25.9%) | 31 (23.3%) | 48 (26.4%) | |||
| Metabolic components | Hypertension | 1100 (81.5%) | 267 (73.6%) | 611 (84.5%) | 5.8 × 10−7+++ | 698 (78.9%) | 124 (63.3%) | 434 (83%) | 402 (86.5%) | 143 (85.6%) | 177 (87.2%) | 3.4 × 10−5+++ |
| Hyperglycemia /DM2 | 804 (59.6%) | 134 (36.9%) | 496 (68.6%) | 2.2 × 10−16+++ | 517 (58.4%) | 67 (34.2%) | 343 (66%) | 287 (61.7%) | 67 (40.1%) | 153 (75.4%) | 0.01+ | |
| Low-HDL | 905 (67%) | 275 (75.6%) | 452 (62.5%) | 9.1 × 10−6+++ | 601 (67.9%) | 153 (78.1%) | 329 (63.3%) | 304 (65.4%) | 122 (73%) | 123 (60.6%) | 0.27 | |
| Hyper- triglyceridemia | 522 (38.7%) | 140 (38.6%) | 264 (36.5%) | 0.97 | 308 (34.8%) | 62 (31.6%) | 172 (33%) | 214 (46%) | 78 (46.7%) | 92 (45.3%) | 3.1 × 10−5+++ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisoni, S.; Marrachelli, V.G.; Morales, J.M.; Maestrini, S.; Di Blasio, A.M.; Monleón, D. Sex Dimorphism in the Metabolome of Metabolic Syndrome in Morbidly Obese Individuals. Metabolites 2022, 12, 419. https://doi.org/10.3390/metabo12050419
Pisoni S, Marrachelli VG, Morales JM, Maestrini S, Di Blasio AM, Monleón D. Sex Dimorphism in the Metabolome of Metabolic Syndrome in Morbidly Obese Individuals. Metabolites. 2022; 12(5):419. https://doi.org/10.3390/metabo12050419
Chicago/Turabian StylePisoni, Serena, Vannina G. Marrachelli, Jose M. Morales, Sabrina Maestrini, Anna M. Di Blasio, and Daniel Monleón. 2022. "Sex Dimorphism in the Metabolome of Metabolic Syndrome in Morbidly Obese Individuals" Metabolites 12, no. 5: 419. https://doi.org/10.3390/metabo12050419
APA StylePisoni, S., Marrachelli, V. G., Morales, J. M., Maestrini, S., Di Blasio, A. M., & Monleón, D. (2022). Sex Dimorphism in the Metabolome of Metabolic Syndrome in Morbidly Obese Individuals. Metabolites, 12(5), 419. https://doi.org/10.3390/metabo12050419