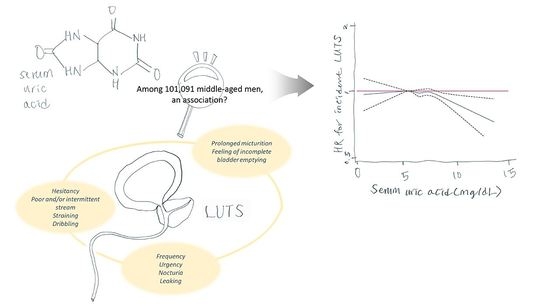
Higher Levels of Serum Uric Acid Have a Significant Association with Lower Incidence of Lower Urinary Tract Symptoms in Healthy Korean Men
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Study Population
2.2. Relationship between SUA Levels and the Incidence of Significant LUTS
2.3. Modifying Effect of Age, Smoking, Alcohol Intake, Physical Activity, and BMI
3. Discussion
4. Materials and Methods
4.1. Participants and Design
4.2. Data Collection and Measurements
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; van Kerrebroeck, P.; Victor, A.; Wein, A. The standardisation of terminology of lower urinary tract function: Report from the Standardisation Sub-committee of the International Continence Society. Am. J. Obstet. Gynecol. 2002, 187, 116–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coyne, K.S.; Sexton, C.C.; Thompson, C.L.; Milsom, I.; Irwin, D.; Kopp, Z.S.; Chapple, C.R.; Kaplan, S.; Tubaro, A.; Aiyer, L.P.; et al. The prevalence of lower urinary tract symptoms (LUTS) in the USA, the UK and Sweden: Results from the Epidemiology of LUTS (EpiLUTS) study. BJU Int. 2009, 104, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.K.; Lee, K.S.; Sumarsono, B.; Kim, S.T.; Kim, H.J.; Lee, H.C.; Kim, S.H. The prevalence of lower urinary tract symptoms in population aged 40 years or over, in South Korea. Investig. Clin. Urol. 2018, 59, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Cambronero Santos, J.; Errando Smet, C. Prevalence of storage lower urinary tract symptoms in male patients attending Spanish urology office. Urinary urgency as predictor of quality of life. Actas Urol. Esp. 2016, 40, 621–627. [Google Scholar] [CrossRef]
- Lee, K.S.; Yoo, T.K.; Liao, L.; Wang, J.; Chuang, Y.C.; Liu, S.P.; Chu, R.; Sumarsono, B. Association of lower urinary tract symptoms and OAB severity with quality of life and mental health in China, Taiwan and South Korea: Results from a cross-sectional, population-based study. BMC Urol. 2017, 17, 108. [Google Scholar] [CrossRef] [Green Version]
- Lepor, H. Pathophysiology of lower urinary tract symptoms in the aging male population. Rev. Urol. 2005, 7 (Suppl. S7), S3–S11. [Google Scholar]
- Lee, C.L.; Kuo, H.C. Current consensus and controversy on the diagnosis of male lower urinary tract symptoms/benign prostatic hyperplasia. Tzu-Chi Med. J. 2017, 29, 6–11. [Google Scholar] [CrossRef]
- De Nunzio, C.; Roehrborn, C.G.; Andersson, K.E.; McVary, K.T. Erectile Dysfunction and Lower Urinary Tract Symptoms. Eur. Urol. Focus 2017, 3, 352–363. [Google Scholar] [CrossRef]
- Li, Z.; Huang, W.; Wang, X.; Zhang, Y. The relationship between lower urinary tract symptoms and irritable bowel syndrome: A meta-analysis of cross-sectional studies. Minerva Urol. Nefrol. 2018, 70, 386–392. [Google Scholar] [CrossRef]
- Barbosa, J.A.; Muracca, E.; Nakano, E.; Paranhos, M.; Natalino, R.; Cordeiro, P.; Srougi, M.; Antunes, A.A. Risk factors for male lower urinary tract symptoms: The role of metabolic syndrome and androgenetic alopecia in a Latin American population. Urology 2013, 82, 182–188. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, Y.W.; Lee, S.W. The Relationships between Thyroid Hormone Levels and Lower Urinary Tract Symptoms/Benign Prostatic Hyperplasia. World J. Mens Health 2019, 37, 364–371. [Google Scholar] [CrossRef]
- Sanford, M.T.; Rodriguez, L.V. The role of environmental stress on lower urinary tract symptoms. Curr. Opin. Urol. 2017, 27, 268–273. [Google Scholar] [CrossRef] [PubMed]
- De Nunzio, C.; Presicce, F.; Tubaro, A. Inflammatory mediators in the development and progression of benign prostatic hyperplasia. Nat. Rev. Urol. 2016, 13, 613–626. [Google Scholar] [CrossRef]
- Kukko, V.; Kaipia, A.; Talala, K.; Taari, K.; Tammela, T.L.J.; Auvinen, A.; Murtola, T.J. Allopurinol and risk of benign prostatic hyperplasia in a Finnish population-based cohort. Prostate Cancer Prostatic Dis. 2018, 21, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Sangkop, F.; Singh, G.; Rodrigues, E.; Gold, E.; Bahn, A. Uric acid: A modulator of prostate cells and activin sensitivity. Mol. Cell Biochem. 2016, 414, 187–199. [Google Scholar] [CrossRef]
- Kanbara, A.; Hakoda, M.; Seyama, I. Urine alkalization facilitates uric acid excretion. Nutr. J. 2010, 9, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Persson, B.E.; Ronquist, G. Evidence for a mechanistic association between nonbacterial prostatitis and levels of urate and creatinine in expressed prostatic secretion. J. Urol. 1996, 155, 958–960. [Google Scholar] [CrossRef]
- Doblado, M.; Moley, K.H. Facilitative glucose transporter 9, a unique hexose and urate transporter. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E831–E835. [Google Scholar] [CrossRef] [Green Version]
- Das Gupta, E.; Sakthiswary, R.; Lee, S.L.; Wong, S.F.; Hussein, H.; Gun, S.C. Clinical significance of SLC2A9/GLUT9 rs11722228 polymorphisms in gout. Int. J. Rheum. Dis. 2018, 21, 705–709. [Google Scholar] [CrossRef]
- Uemura, N.; Murakami, R.; Chiba, Y.; Yanase, K.; Fujihara, R.; Mashima, M.; Matsumoto, K.; Kawauchi, M.; Shirakami, G.; Ueno, M. Immunoreactivity of urate transporters, GLUT9 and URAT1, is located in epithelial cells of the choroid plexus of human brains. Neurosci. Lett. 2017, 659, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Song, J.U.; Hwang, J.; Ahn, J.K. Serum uric acid is positively associated with pulmonary function in Korean health screening examinees. Mod. Rheumatol. 2017, 27, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Hwang, J.H.; Ryu, S.; Ahn, J.K. Higher serum uric acid is associated with higher lumbar spine bone mineral density in male health-screening examinees: A cross-sectional study. J. Bone Miner. Metab. 2019, 37, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Hwang, J.H.; Chung, S.M.; Kwon, M.J.; Ahn, J.K. Association between serum uric acid and arterial stiffness in a low-risk, middle-aged, large Korean population: A cross-sectional study. Medicine 2018, 97, e12086. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Yang, Y.J.; Kim, B.S.; Kang, J.H. Validity and Reliability of Korean Version of International Physical Activity Questionnaire (IPAQ) Short Form. Korean J. Fam. Med. 2007, 28, 532–541. [Google Scholar]
- Kim, M.K.; Lee, W.Y.; Kang, J.H.; Kang, J.H.; Kim, B.T.; Kim, S.M.; Kim, E.M.; Suh, S.H.; Shin, H.J.; Lee, K.R.; et al. 2014 clinical practice guidelines for overweight and obesity in Korea. Endocrinol. Metab. 2014, 29, 405–409. [Google Scholar] [CrossRef] [Green Version]
- Park, H.J.; Park, C.H.; Chang, Y.; Ryu, S. Sitting time, physical activity and the risk of lower urinary tract symptoms: A cohort study. BJU Int. 2018, 122, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.C. Validation of Korean Version of International Prostate Symptom Score: A Comparison of Physician versus Self-administration. Korean J. Androl. 2007, 25, 32–35. [Google Scholar]


| Characteristics | Overall | Uric Acid Level (mg/dL) | p for Trend | |||||
|---|---|---|---|---|---|---|---|---|
| <5.5 | 5.5–6.4 | 6.5–7.4 | 7.5–8.4 | 8.5–9.4 | ≥9.5 | |||
| Number | 101,091 | 26,926 | 35,153 | 25,230 | 10,105 | 2809 | 868 | |
| Age, y | 38.1 ± 6.8 | 39.2 ± 7.3 | 38.1 ± 6.7 | 37.5 ± 6.4 | 36.9 ± 6.1 | 36.6 ± 5.8 | 36.1 ± 5.7 | <0.001 |
| Obesity (%) | 39.6 | 27.6 | 35.3 | 46.7 | 58.5 | 67.3 | 74.4 | <0.001 |
| Current smoker (%) | 36.7 | 37.5 | 36.5 | 36.6 | 36.5 | 35.0 | 33.1 | 0.001 |
| Alcohol intake (%) 1 | 33.5 | 31.7 | 32.3 | 35.0 | 37.2 | 38.4 | 41.5 | <0.001 |
| HEPA (%) | 16.3 | 17.6 | 16.2 | 15.7 | 15.3 | 15.3 | 17.3 | <0.001 |
| High education level (%) 2 | 89.8 | 88.3 | 90.0 | 90.7 | 90.6 | 90.0 | 89.0 | <0.001 |
| History of diabetes (%) | 1.9 | 3.3 | 1.6 | 1.2 | 1.0 | 0.9 | 0.5 | <0.001 |
| History of hypertension (%) | 5.0 | 4.0 | 4.5 | 5.4 | 7.1 | 9.0 | 10.7 | <0.001 |
| BMI, kg/m2 | 24.5 ± 3.0 | 23.6 ± 2.7 | 24.2 ± 2.7 | 25.0 ± 2.9 | 25.8 ± 3.0 | 26.6 ± 3.3 | 27.3 ± 3.4 | <0.001 |
| Systolic BP, mmHg | 114.3 ± 11.4 | 112.5 ± 11.2 | 113.6 ± 11.1 | 115.3 ± 11.3 | 117.0 ± 11.8 | 118.8 ± 11.8 | 121.1 ± 12.3 | <0.001 |
| Diastolic BP, mmHg | 73.6 ± 9.4 | 72.3 ± 9.1 | 73.1 ± 9.2 | 74.4 ± 9.4 | 75.6 ± 9.7 | 77.1 ± 9.6 | 78.8 ± 10.4 | <0.001 |
| Glucose, mg/dL | 96.9 ± 14.4 | 98.3 ± 19.8 | 96.3 ± 12.5 | 96.3 ± 11.1 | 96.6 ± 11.3 | 97.5 ± 11.4 | 96.4 ± 10.6 | <0.001 |
| Creatinine, mg/dL | 0.98 ± 0.12 | 0.95 ± 0.11 | 0.98 ± 0.11 | 0.99 ± 0.12 | 1.01 ± 0.12 | 1.03 ± 0.12 | 1.06 ± 0.13 | <0.001 |
| eGFR, mL/min/1.73 m2 | 93.1 ± 13.3 | 95.8 ± 13.8 | 93.5 ± 13.4 | 91.8 ± 13.4 | 90.1 ± 13.5 | 88.3 ± 13.2 | 85.7 ± 13.3 | <0.001 |
| Total cholesterol, mg/dL | 199.9 ± 34.3 | 193.2 ± 32.8 | 198.1 ± 33.3 | 203.7 ± 34.4 | 208.6 ± 35.3 | 214.3 ± 36.9 | 217.5 ± 39.5 | <0.001 |
| LDL cholesterol, mg/dL | 128.5 ± 31.3 | 122.0 ± 30.0 | 127.1 ± 30.5 | 132.3 ± 31.5 | 136.4 ± 32.2 | 140.7 ± 33.2 | 141.3 ± 33.8 | <0.001 |
| HDL cholesterol, mg/dL | 52.9 ± 12.8 | 55.4 ± 13.2 | 53.5 ± 12.7 | 51.5 ± 12.2 | 49.7 ± 11.7 | 48.6 ± 11.4 | 47.6 ± 11.0 | <0.001 |
| Triglycerides, mg/dL | 113 (80–163) | 98 (72–139) | 108 (78–153) | 123 (87–176) | 138 (96–199) | 155 (106–223) | 167 (115–243) | <0.001 |
| PSA, ng/dL | 0.82 (0.59–1.13) | 0.82 (0.59–1.14) | 0.82 (0.60–1.13) | 0.81 (0.59–1.13) | 0.80 (0.58–1.12) | 0.79 (0.57–1.09) | 0.80 (0.56–1.11) | <0.001 |
| HOMA-IR | 1.29 (0.86–1.90) | 1.12 (0.76–1.62) | 1.24 (0.83–1.80) | 1.40 (0.94–2.04) | 1.59 (1.05–2.31) | 1.78 (1.19–2.61) | 1.83 (1.19–2.73) | <0.001 |
| hsCRP, mg/L | 0.5 (0.3–1.0) | 0.4 (0.3–0.8) | 0.5 (0.3–0.9) | 0.6 (0.3–1.1) | 0.7 (0.4–1.4) | 0.8 (0.5–1.6) | 1.0 (0.6–1.9) | <0.001 |
| Total calorie intake, kcal/d 3 | 1636.6 (1306.5–2023.4) | 1640.3 (1313.5–2017.0) | 1636.9 (1315.4–2012.9) | 1636.2 (1302.5–2033.2) | 1630.3 (1281.6–2040.9) | 1636.1 (1282.2–2066.4) | 1608.6 (1262.9–2075.5) | 0.448 |
| Uric Acid Level (mg/dL) | Person Years | Incident Case | Incidence Density (per 1000 Person Years) | Age-Adjusted HR 1 (95% CI) | Multivariate-Adjusted HR 1 (95% CI) | |
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||
| <5.5 | 96,895.1 | 3994 | 41.2 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 5.5–6.4 | 125,703.0 | 4715 | 37.5 | 0.98 (0.94–1.02) | 0.99 (0.95–1.03) | 1.00 (0.96–1.05) |
| 6.5–7.4 | 88,701.7 | 3134 | 35.3 | 0.96 (0.92–1.01) | 0.97 (0.92–1.02) | 1.00 (0.95–1.06) |
| 7.5–8.4 | 35,049.3 | 1216 | 34.5 | 0.98 (0.92–1.04) | 0.99 (0.93–1.06) | 1.03 (0.96–1.11) |
| 8.5–9.4 | 9626.9 | 291 | 30.2 | 0.88 (0.78–0.99) | 0.90 (0.80–1.02) | 0.98 (0.86–1.12) |
| ≥9.5 | 3006.6 | 74 | 24.6 | 0.73 (0.58–0.92) | 0.74 (0.58–0.93) | 0.77 (0.59–0.99) |
| p for trend | 0.008 | 0.039 | 0.850 | |||
| Subgroup | Uric Acid Level (mg/dL) | p for Trend | p for Interaction | |||||
|---|---|---|---|---|---|---|---|---|
| <5.5 | 5.5–6.4 | 6.5–7.4 | 7.5–8.4 | 8.5–9.4 | ≥9.5 | |||
| Age, y | 0.261 | |||||||
| <50 (n = 95,408) | 1.00 | 0.94 (0.90–0.99) | 0.92 (0.87–0.97) | 0.91 (0.84–0.98) | 0.84 (0.74–0.96) | 0.65 (0.50–0.84) | <0.001 | |
| ≥50 (n = 5683) | 1.00 | 1.11 (0.94–1.31) | 0.90 (0.72–1.11) | 1.19 (0.87–1.63) | 0.78 (0.39–1.58) | 0.687 | ||
| Smoking | 0.916 | |||||||
| Never (n = 61,406) | 1.00 | 1.01 (0.95–1.08) | 1.00 (0.93–1.07) | 1.03 (0.94–1.14) | 0.95 (0.80–1.14) | 0.73 (0.52–1.04) | 0.619 | |
| Smoker (n = 35,611) | 1.00 | 0.97 (0.90–1.05) | 0.97 (0.89–1.05) | 0.99 (0.88–1.11) | 1.02 (0.83–1.25) | 0.82 (0.55–1.21) | 0.581 | |
| Alcohol intake, g/day | 0.534 | |||||||
| <20 (n = 66,029) | 1.00 | 0.98 (0.92–1.04) | 0.97 (0.91–1.04) | 1.02 (0.93–1.12) | 0.90 (0.75–1.07) | 0.73 (0.52–1.04) | 0.289 | |
| ≥20 (n = 33,314) | 1.00 | 1.05 (0.97–1.14) | 1.05 (0.96–1.15) | 1.07 (0.95–1.20) | 1.11 (0.91–1.35) | 0.83 (0.57–1.21) | 0.353 | |
| HEPA | 0.859 | |||||||
| No (n = 84,021) | 1.00 | 0.99 (0.94–1.05) | 1.00 (0.94–1.06) | 1.04 (0.96–1.13) | 1.00 (0.87–1.15) | 0.76 (0.57–1.00) | 0.985 | |
| Yes (n = 16,412) | 1.00 | 1.05 (0.94–1.18) | 1.01 (0.89–1.16) | 0.99 (0.82–1.19) | 0.90 (0.63–1.28) | 0.82 (0.44–1.54) | 0.621 | |
| BMI (kg/m2) | 0.182 | |||||||
| <25 (n = 61,019) | 1.00 | 1.03 (0.97–1.09) | 1.00 (0.94–1.07) | 1.02 (0.91–1.13) | 1.04 (0.84–1.29) | 1.10 (0.71–1.69) | 0.689 | |
| ≥25 (n = 40,072) | 1.00 | 0.94 (0.86–1.03) | 0.98 (0.89–1.07) | 1.02 (0.91–1.13) | 0.92 (0.78–1.10) | 0.64 (0.46–0.88) | 0.389 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.; Ryu, S.; Ahn, J.K. Higher Levels of Serum Uric Acid Have a Significant Association with Lower Incidence of Lower Urinary Tract Symptoms in Healthy Korean Men. Metabolites 2022, 12, 649. https://doi.org/10.3390/metabo12070649
Hwang J, Ryu S, Ahn JK. Higher Levels of Serum Uric Acid Have a Significant Association with Lower Incidence of Lower Urinary Tract Symptoms in Healthy Korean Men. Metabolites. 2022; 12(7):649. https://doi.org/10.3390/metabo12070649
Chicago/Turabian StyleHwang, Jiwon, Seungho Ryu, and Joong Kyong Ahn. 2022. "Higher Levels of Serum Uric Acid Have a Significant Association with Lower Incidence of Lower Urinary Tract Symptoms in Healthy Korean Men" Metabolites 12, no. 7: 649. https://doi.org/10.3390/metabo12070649
APA StyleHwang, J., Ryu, S., & Ahn, J. K. (2022). Higher Levels of Serum Uric Acid Have a Significant Association with Lower Incidence of Lower Urinary Tract Symptoms in Healthy Korean Men. Metabolites, 12(7), 649. https://doi.org/10.3390/metabo12070649