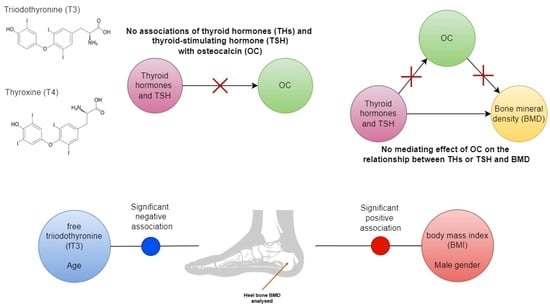
Thyroid Hormones Are Not Associated with Plasma Osteocalcin Levels in Adult Population with Normal Thyroid Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Biochemical and Body Measurements
2.3. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zofkova, I. Bone tissue as a systemic endocrine regulator. Physiol. Res. 2015, 64, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.B.; O’Shea, P.J.; Scott, A.J.; Robson, H.; Siebler, T.; Shalet, S.M.; Samarut, J.; Chassande, O.; Williams, G.R. Molecular Mechanisms of Thyroid Hormone Effects on Bone Growth and Function. Mol. Genet. Metab. 2002, 75, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Williams, G.R. The thyroid and the skeleton. Clin. Endocrinol. 2004, 61, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Tsourdi, E.; Wallaschofski, H.; Rauner, M.; Nauck, M.; Pietzner, M.; Rettig, R.; Ittermann, T.; Völzke, H.; Völker, U.; Hofbauer, L.C.; et al. Thyrotropin serum levels are differentially associated with biochemical markers of bone turnover and stiffness in women and men: Results from the SHIP cohorts. Osteoporos. Int. 2015, 27, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Xing, W.; Pourteymoor, S.; Mohan, S. Effects of Thyroxine (T4), 3,5,3′-triiodo-l-thyronine (T3) and their Metabolites on Osteoblast Differentiation. Calcif. Tissue Int. 2016, 99, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Siru, R.; Alfonso, H.; Chubb, S.A.P.; Golledge, J.; Flicker, L.; Yeap, B.B. Subclinical thyroid dysfunction and circulating thyroid hormones are not associated with bone turnover markers or incident hip fracture in older men. Clin. Endocrinol. 2018, 89, 93–99. [Google Scholar] [CrossRef]
- Abe, E.; Marians, R.C.; Yu, W.; Wu, X.-B.; Ando, T.; Li, Y.; Iqbal, J.; Eldeiry, L.; Rajendren, G.; Blair, H.C.; et al. TSH Is a Negative Regulator of Skeletal Remodeling. Cell 2003, 115, 151–162. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Chen, R.; Jiang, Y.; Yang, Y.; He, L.; Luo, C.; Dong, J.; Rong, L. A meta-analysis of serum osteocalcin level in postmenopausal osteoporotic women compared to controls. BMC Musculoskelet. Disord. 2019, 20, 532–537. [Google Scholar] [CrossRef]
- Ducy, P.; Desbois, C.; Boyce, B.; Pinero, G.; Story, B.; Dunstan, C.; Smith, E.; Bonadio, J.; Goldstein, S.; Gundberg, C.; et al. Increased bone formation in osteocalcin-deficient mice. Nature 1996, 382, 448–452. [Google Scholar] [CrossRef] [Green Version]
- Kondo, A.; Otsuka, T.; Kato, K.; Matsushima-Nishiwaki, R.; Kuroyanagi, G.; Mizutani, J.; Tokuda, H.; Kozawa, O. AMP-activated protein kinase regulates thyroid hormone-stimulated osteocalcin synthesis in osteoblasts. Int. J. Mol. Med. 2013, 31, 1457–1462. [Google Scholar] [CrossRef]
- Fujita, K.; Tokuda, H.; Kainuma, S.; Kuroyanagi, G.; Yamamoto, N.; Matsushima-Nishiwaki, R.; Harada, A.; Kozawa, O.; Otsuka, T. Resveratrol suppresses thyroid hormone-induced osteocalcin synthesis in osteoblasts. Mol. Med. Rep. 2017, 16, 2881–2886. [Google Scholar] [CrossRef] [Green Version]
- Rudan, I.; Marusic, A.; Jankovic, S.; Rotim, K.; Boban, M.; Lauc, G.; Grković, I.; Ðogaš, Z.; Zemunik, T.; Vatavuk, Z. “10001 Dalmatians:” Croatia launches its national biobank. Croat. Med. J. 2009, 50, 4–6. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2016. [Google Scholar]
- Patil, I. Visualizations with statistical details: The ’ggstatsplot’ approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Nie, X.; Xu, Y.; Shen, Y.; Wang, Y.; Ma, X.; Bao, Y. Suppressing Effect of Free Triiodothyronine on the Negative Association between Body Mass Index and Serum Osteocalcin Levels in Euthyroid Population. Int. J. Endocrinol. 2021, 2021, 6624516. [Google Scholar] [CrossRef]
- Murphy, E.; Glüer, C.C.; Reid, D.M.; Felsenberg, D.; Roux, C.; Eastell, R.; Williams, G.R. Thyroid Function within the Upper Normal Range Is Associated with Reduced Bone Mineral Density and an Increased Risk of Nonvertebral Fractures in Healthy Euthyroid Postmenopausal Women. J. Clin. Endocrinol. Metab. 2010, 95, 3173–3181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Deure, W.M.; Uitterlinden, A.G.; Hofman, A.; Rivadeneira, F.; Pols, H.A.P.; Peeters, R.P.; Visser, T.J. Effects of serum TSH and FT4 levels and the TSHR-Asp727Glu polymorphism on bone: The Rotterdam Study. Clin. Endocrinol. 2008, 68, 175–181. [Google Scholar] [CrossRef]
- Hwangbo, Y.; Kim, J.H.; Kim, S.W.; Park, Y.J.; Shin, C.S.; Cho, N.H. High-normal free thyroxine levels are associated with low trabecular bone scores in euthyroid postmenopausal women. Osteoporos. Int. 2015, 27, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Zhang, W.; Zhang, Y.; Zhang, M.; Huan, Z.; Yu, C.; Zhang, X.; Wang, Y.; Xu, J. Thyroid-stimulating hormone decreases the risk of osteoporosis by regulating osteoblast proliferation and differentiation. BMC Endocr. Disord. 2021, 21, 49. [Google Scholar] [CrossRef]
- Kim, D.J.; Khang, Y.H.; Koh, J.M.; Shong, Y.K.; Kim, G.S. Low normal TSH levels are associated with low bone mineral density in healthy postmenopausal women. Clin. Endocrinol. 2006, 64, 86–90. [Google Scholar] [CrossRef]
- Waneen, W.S.; Francis, K.L.; Priscilla, G.M. Physical Dimensions of Aging; Human Kinetics: New York, NY, USA, 2005. [Google Scholar]
- Lee, E.Y.; Kim, D.; Kim, K.M.; Kim, K.J.; Choi, H.S.; Rhee, Y.; Lim, S.-K. Age-Related Bone Mineral Density Patterns in Koreans (KNHANES IV). J. Clin. Endocrinol. Metab. 2012, 97, 3310–3318. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Park, S.-K. Differences in Physical Characteristics of the Lower Extremity and Running Biomechanics between Different Age Groups. Int. J. Environ. Res. Public Health 2022, 19, 4320. [Google Scholar] [CrossRef] [PubMed]
- Cvijetic Avdagic, S.; Colic Baric, I.; Keser, I.; Rumbak, I.; Satalic, Z. Influence of heredity and environment on peak bone density: A review of studies in Croatia. Arh. Hig. Rada Toksikol. 2012, 63 (Suppl. S1), 11–16. [Google Scholar] [PubMed]
- Warming, L.; Hassager, C.; Christiansen, C. Changes in bone mineral density with age in men and women: A longitudinal study. Osteoporos. Int. 2002, 13, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Ertungealp, E.; Seyisoglu, H.; Erel, C.T.; Senturk, L.M.; Gezer, A. Changes in bone mineral density with age, menopausal status and body mass index in Turkish women. Climacteric 1999, 2, 45–51. [Google Scholar] [CrossRef]



| Variable | Total (N = 694) | Reference Interval |
|---|---|---|
| Women | 396 (57.1%) | - |
| Age | 51.6 (14.8) | - |
| OC | 15.6 (13–18.5) | 5–25 ng/mL |
| CT | 5.2 (2.68–8.2) | 0–20 ng/mL |
| TSH | 1.43 (1.05–2.01) | 0.3–3.6 mIU/L |
| fT3 | 4.57 (0.49) | 3.39–6.47 pmol/L |
| fT4 | 12.7 (11.9–13.9) | 10.29–21.88 pmol/L |
| fT3/fT4 | 0.36 (0.05) | - |
| TgAb | 7.6 (5–11.2) | 5–100 IU/mL |
| TPOAb | 2.7 (1.3–6.3) | 1–16 IU/mL |
| PTH | 21.5 (5.7) | 12.26–35.5 pg/ml |
| Total serum Calcium | 2.36 (0.1) | 2.14–2.53 mmol/L |
| BMI | 27.31 (4.34) | 18.5–24.9 |
| Absolute BMD | 0.47 (0.1) (g/cm2) | - |
| Variable | OC | TSH | fT3 | fT4 | fT3/fT4 | BMI | BMD |
|---|---|---|---|---|---|---|---|
| OC | - | - | - | - | - | - | - |
| TSH | −0.01 | - | - | - | - | - | - |
| fT3 | −0.02 | −0.005 | - | - | - | - | - |
| fT4 | −0.004 | −0.08 | 0.28 * | - | - | - | - |
| fT3/fT4 | −0.01 | 0.07 | 0.31 * | −0.45 * | - | - | - |
| BMI | −0.09 | −0.023 | −0.043 | −0.065 | 0.021 | - | - |
| BMD | −0.05 | −0.023 | −0.077 | 0.026 | −0.101 | 0.204 * | - |
| Variable | Premenopausal Women (N = 199) | Postmenopausal Women (N = 197) | p-Value |
|---|---|---|---|
| Age | 40.2 (10.98) | 61.7 (8.5) | <0.001 * |
| OC (ng/mL) | 15.45 (12.6–18.43) | 16.6 (13.1–19.9) | 0.044 * |
| TSH (mIU/L) | 1.52 (1.15–2.85) | 1.41 (1.08–2.01) | 0.294 |
| fT3 (pmol/L) | 4.6 (0.4) | 4.5 (0.48) | 0.094 |
| fT4 (pmol/L) | 12.7 (11.9–13.9) | 12.3 (11.7–13.5) | 0.223 |
| BMI | 26.64 (4.3) | 28.32 (4.0) | <0.001 * |
| Absolute BMD (g/cm2) | 0.46 (0.1) | 0.39 (0.1) | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pleić, N.; Brdar, D.; Gunjača, I.; Babić Leko, M.; Torlak, V.; Punda, A.; Polašek, O.; Hayward, C.; Zemunik, T. Thyroid Hormones Are Not Associated with Plasma Osteocalcin Levels in Adult Population with Normal Thyroid Function. Metabolites 2022, 12, 719. https://doi.org/10.3390/metabo12080719
Pleić N, Brdar D, Gunjača I, Babić Leko M, Torlak V, Punda A, Polašek O, Hayward C, Zemunik T. Thyroid Hormones Are Not Associated with Plasma Osteocalcin Levels in Adult Population with Normal Thyroid Function. Metabolites. 2022; 12(8):719. https://doi.org/10.3390/metabo12080719
Chicago/Turabian StylePleić, Nikolina, Dubravka Brdar, Ivana Gunjača, Mirjana Babić Leko, Vesela Torlak, Ante Punda, Ozren Polašek, Caroline Hayward, and Tatijana Zemunik. 2022. "Thyroid Hormones Are Not Associated with Plasma Osteocalcin Levels in Adult Population with Normal Thyroid Function" Metabolites 12, no. 8: 719. https://doi.org/10.3390/metabo12080719
APA StylePleić, N., Brdar, D., Gunjača, I., Babić Leko, M., Torlak, V., Punda, A., Polašek, O., Hayward, C., & Zemunik, T. (2022). Thyroid Hormones Are Not Associated with Plasma Osteocalcin Levels in Adult Population with Normal Thyroid Function. Metabolites, 12(8), 719. https://doi.org/10.3390/metabo12080719