The Exploration of Fetal Growth Restriction Based on Metabolomics: A Systematic Review
Abstract
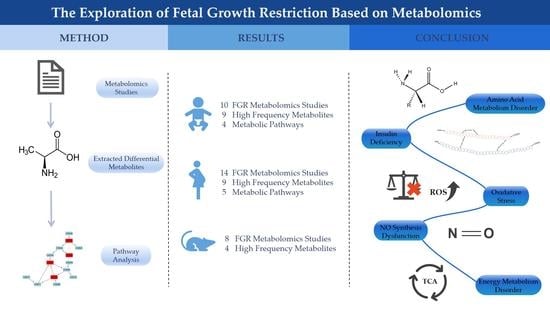
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
3.1. Human Neonatal Research
3.1.1. Study Characteristics of Human Neonatal Research
3.1.2. Analysis of High Frequency Biomarkers in Human Neonatal Research
| Neonatal Studies | ||||||
| No | Metabolites | Total Hits | Up | Down | ||
| Hits | Bio-Specimen | Hits | Bio-Specimen | |||
| 1 | Alanine | 5 | 3 | Cord serum [19] Dried blood [26] Cord plasma [28] | 2 | Cord serum [24] (l) Cord plasma [25] |
| 2 | Proline | 4 | 3 | Cord serum [21] Dried blood [26] Cord plasma [28] | 1 | Cord plasma [25] |
| 3 | Valine | 4 | 2 | Cord serum [19,21] | 2 | Cord serum [24] (l) Dried blood [26] |
| 4 | Phenylalanine | 4 | 2 | Cord serum [21] Cord plasma [25] | 2 | Cord serum [19,24] (e) |
| 5 | Glutamine | 4 | 2 | Cord serum [24] (e) Cord plasma [28] | 2 | Cord serum [24] (l) Cord plasma [25] |
| 6 | Isoleucine | 3 | 2 | Cord serum [19,21] | 1 | Cord plasma [20] |
| 7 | Creatine | 3 | 2 | Cord serum [24] (e) Urine [27] | 1 | Cord serum [22] |
| 8 | Tryptophan | 3 | 1 | Cord serum [21] | 2 | Cord serum [19] Cord plasma [28] |
| 9 | Choline | 3 | - | - | 3 | Cord serum [22,24] (e l) Cord plasma [25] |
| Maternal Studies | ||||||
| No | Metabolites | Total Hits | Up | Down | ||
| Hits | Bio-Specimen | Hits | Bio-Specimen | |||
| 1 | Alanine | 5 | 1 | Maternal serum [19] | 4 | Urine [29] Maternal hair [30] Maternal plasma [23] Placenta [31] |
| 2 | Citrate | 4 | 2 | Placenta [31] Human-milk [32] | 2 | Urine [29] Maternal plasma [23] |
| 3 | Valine | 4 | 1 | Maternal serum [19] | 3 | Maternal hair [30] Placenta [31] Human-milk [32] |
| 4 | Glycine | 4 | - | - | 4 | Urine [29] Maternal hair [30] Placenta [28,31] |
| 5 | Isoleucine | 3 | 1 | Maternal serum [19] | 2 | Maternal hair [30] Human-milk [32] |
| 6 | Lactate | 3 | 1 | Placenta [33] | 2 | Urine [29] Maternal hair [30] |
| 7 | Tyrosine | 3 | - | - | 3 | Urine [29] Maternal hair [30] Placenta [28] |
| 8 | Aspartate | 3 | - | - | 3 | Maternal hair [30] Placenta [28,31] |
| 9 | 3-Hydroxybutyrate | 3 | - | - | 3 | Maternal serum [19,34] Placenta [31] |
3.1.3. Metabolic Pathway Analysis of Potential Biomarkers in Human Neonatal Research
3.2. Human Maternal Research
3.2.1. Study Characteristics of Human Maternal Research
3.2.2. Analysis of High Frequency Biomarkers in Human Maternal Research
3.2.3. Metabolic Pathway Analysis of Potential Biomarkers in Human Maternal Research
3.3. Comparison between Human Neonatal and Maternal Research
3.4. Classification Potential of the Metabolic Biomarkers
3.5. Animal Studies
3.5.1. Study Characteristics of Animal Model Research
3.5.2. Analysis of High Frequency Biomarkers in Animal Research
4. Discussion
4.1. Potential Metabolic Dysregulations of FGR
4.1.1. Amino Acid Metabolism Disorder
4.1.2. Insulin Deficiency
4.1.3. Oxidative Stress
4.1.4. NO Synthesis Dysfunction
4.1.5. Energy Metabolism Disorder
4.2. Reliability of Metabolic Biomarkers/Metabolite Panels
4.3. Limitations of Current Metabolomics Studies on FGR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nardozza, L.M.; Caetano, A.C.; Zamarian, A.C.; Mazzola, J.B.; Silva, C.P.; Marcal, V.M.; Lobo, T.F.; Peixoto, A.B.; Araujo Junior, E. Fetal growth restriction: Current knowledge. Arch. Gynecol. Obs. 2017, 295, 1061–1077. [Google Scholar] [CrossRef] [PubMed]
- Infants with Fetal (Intrauterine) Growth Restriction. Available online: https://www.uptodate.com/contents/infants-with-fetal-intrauterine-growth-restriction#:~:text=Infants%20with%20fetal%20growth%20rstriction,with%20normal%20in%20utero%20growth (accessed on 1 September 2022).
- Deter, R.L.; Lee, W.; Sangi-Haghpeykar, H.; Tarca, A.L.; Yeo, L.; Romero, R. Fetal growth cessation in late pregnancy: Its impact on predicted size parameters used to classify small for gestational age neonates. J. Matern. Fetal Neonatal Med. 2015, 28, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Melamed, N.; Baschat, A.; Yinon, Y.; Athanasiadis, A.; Mecacci, F.; Figueras, F.; Berghella, V.; Nazareth, A.; Tahlak, M.; McIntyre, H.D.; et al. FIGO (international Federation of Gynecology and obstetrics) initiative on fetal growth: Best practice advice for screening, diagnosis, and management of fetal growth restriction. Int. J. Gynaecol. Obs. 2021, 152 (Suppl. 1), 3–57. [Google Scholar] [CrossRef]
- Society for Maternal-Fetal Medicine; Martins, J.G.; Biggio, J.R.; Abuhamad, A. Society for Maternal-Fetal Medicine Consult Series #52: Diagnosis and management of fetal growth restriction: (Replaces Clinical Guideline Number 3, April 2012). Am. J. Obs. Gynecol. 2020, 223, B2–B17. [Google Scholar] [CrossRef]
- Dessi, A.; Ottonello, G.; Fanos, V. Physiopathology of intrauterine growth retardation: From classic data to metabolomics. J. Matern.Fetal Neonatal Med. 2012, 25, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Fung, C.; Zinkhan, E. Short- and Long-Term Implications of Small for Gestational Age. Obs. Gynecol. Clin. N. Am. 2021, 48, 311–323. [Google Scholar] [CrossRef]
- Crispi, F.; Miranda, J.; Gratacos, E. Long-term cardiovascular consequences of fetal growth restriction: Biology, clinical implications, and opportunities for prevention of adult disease. Am. J. Obs. Gynecol. 2018, 218, S869–S879. [Google Scholar] [CrossRef]
- Valsamakis, G.; Kanaka-Gantenbein, C.; Malamitsi-Puchner, A.; Mastorakos, G. Causes of intrauterine growth restriction and the postnatal development of the metabolic syndrome. Ann. N. Y. Acad. Sci. 2006, 1092, 138–147. [Google Scholar] [CrossRef]
- Pallotto, E.K.; Kilbride, H.W. Perinatal outcome and later implications of intrauterine growth restriction. Clin. Obstet. Gynecol. 2006, 49, 257–269. [Google Scholar] [CrossRef]
- Robert Peter, J.; Ho, J.J.; Valliapan, J.; Sivasangari, S. Symphysial fundal height (SFH) measurement in pregnancy for detecting abnormal fetal growth. Cochrane Database Syst. Rev. 2015, CD008136. [Google Scholar] [CrossRef]
- Lees, C.C.; Stampalija, T.; Baschat, A.; da Silva Costa, F.; Ferrazzi, E.; Figueras, F.; Hecher, K.; Kingdom, J.; Poon, L.C.; Salomon, L.J.; et al. ISUOG Practice Guidelines: Diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet. Gynecol. 2020, 56, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Shastri, S.; Farahbakhsh, N.; Sharma, P. Intrauterine growth restriction—Part 1. J. Matern. Fetal Neonatal Med. 2016, 29, 3977–3987. [Google Scholar] [CrossRef] [PubMed]
- ACOG Practice Bulletin No. 204: Fetal Growth Restriction. Obstet. Gynecol. 2019, 133, e97–e109. [CrossRef] [PubMed]
- Bujak, R.; Struck-Lewicka, W.; Markuszewski, M.J.; Kaliszan, R. Metabolomics for laboratory diagnostics. J. Pharm. Biomed. Anal. 2015, 113, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Metabolomics for Investigating Physiological and Pathophysiological Processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ Clin. Res. Ed. 2009, 339, b2700. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Moros, G.; Boutsikou, T.; Fotakis, C.; Iliodromiti, Z.; Sokou, R.; Katsila, T.; Xanthos, T.; Iacovidou, N.; Zoumpoulakis, P. Insights into intrauterine growth restriction based on maternal and umbilical cord blood metabolomics. Sci. Rep. 2021, 11, 7824. [Google Scholar] [CrossRef]
- Youssef, L.; Simoes, R.V.; Miranda, J.; Garcia-Martin, M.L.; Paules, C.; Crovetto, F.; Amigo, N.; Canellas, N.; Gratacos, E.; Crispi, F. Paired maternal and fetal metabolomics reveal a differential fingerprint in preeclampsia versus fetal growth restriction. Sci. Rep. 2021, 11, 14422. [Google Scholar] [CrossRef]
- Favretto, D.; Cosmi, E.; Ragazzi, E.; Visentin, S.; Tucci, M.; Fais, P.; Cecchetto, G.; Zanardo, V.; Viel, G.; Ferrara, S.D. Cord blood metabolomic profiling in intrauterine growth restriction. Anal. Bioanal. Chem. 2012, 402, 1109–1121. [Google Scholar] [CrossRef]
- Bahado-Singh, R.O.; Yilmaz, A.; Bisgin, H.; Turkoglu, O.; Kumar, P.; Sherman, E.; Mrazik, A.; Odibo, A.; Graham, S.F. Artificial intelligence and the analysis of multi-platform metabolomics data for the detection of intrauterine growth restriction. PLoS ONE 2019, 14, e0214121. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.; Simoes, R.V.; Paules, C.; Canueto, D.; Pardo-Cea, M.A.; Garcia-Martin, M.L.; Crovetto, F.; Fuertes-Martin, R.; Domenech, M.; Gomez-Roig, M.D.; et al. Metabolic profiling and targeted lipidomics reveals a disturbed lipid profile in mothers and fetuses with intrauterine growth restriction. Sci. Rep. 2018, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Cortes, M.; Carbajo, R.J.; Crispi, F.; Figueras, F.; Pineda-Lucena, A.; Gratacos, E. Metabolomic Profile of Umbilical Cord Blood Plasma from Early and Late Intrauterine Growth Restricted (IUGR) Neonates with and without Signs of Brain Vasodilation. PLoS ONE 2013, 8, e80121. [Google Scholar] [CrossRef]
- Ivorra, C.; García-Vicent, C.; Chaves, F.J.; Monleón, D.; Morales, J.M.; Lurbe, E. Metabolomic profiling in blood from umbilical cords of low birth weight newborns. J. Trans. Med. 2012, 10, 142. [Google Scholar] [CrossRef]
- Schupper, A.; Almashanu, S.; Coster, D.; Keidar, R.; Betser, M.; Sagiv, N.; Bassan, H. Metabolic biomarkers of small and large for gestational age newborns. Early Hum. Dev. 2021, 160, 105422. [Google Scholar] [CrossRef] [PubMed]
- Dessì, A.; Marincola, F.C.; Pattumelli, M.G.; Ciccarelli, S.; Corbu, S.; Ossicini, C.; Fanos, V.; Agostino, R. Investigation of the ¹H-NMR based urine metabolomic profiles of IUGR, LGA and AGA newborns on the first day of life. J. Matern. Fetal Neonatal Med. 2014, 27 (Suppl. 2), 13–19. [Google Scholar] [CrossRef]
- Chao de la Barca, J.M.; Chabrun, F.; Lefebvre, T.; Roche, O.; Huetz, N.; Blanchet, O.; Legendre, G.; Simard, G.; Reynier, P.; Gascoin, G. A Metabolomic Profiling of Intra-Uterine Growth Restriction in Placenta and Cord Blood Points to an Impairment of Lipid and Energetic Metabolism. Biomedicines 2022, 10, 1411. [Google Scholar] [CrossRef]
- Maitre, L.; Fthenou, E.; Athersuch, T.; Coen, M.; Toledano, M.B.; Holmes, E.; Kogevinas, M.; Chatzi, L.; Keun, H.C. Urinary metabolic profiles in early pregnancy are associated with preterm birth and fetal growth restriction in the Rhea mother-child cohort study. BMC Med. 2014, 12, 14. [Google Scholar] [CrossRef]
- Sulek, K.; Han, T.L.; Villas-Boas, S.G.; Wishart, D.S.; Soh, S.E.; Kwek, K.; Gluckman, P.D.; Chong, Y.S.; Kenny, L.C.; Baker, P.N. Hair Metabolomics: Identification of Fetal Compromise Provides Proof of Concept for Biomarker Discovery. Theranostics 2014, 4, 953–959. [Google Scholar] [CrossRef]
- Bahado-Singh, R.O.; Turkoglu, O.; Yilmaz, A.; Kumar, P.; Zeb, A.; Konda, S.; Sherman, E.; Kirma, J.; Allos, M.; Odibo, A.; et al. Metabolomic identification of placental alterations in fetal growth restriction. J. Matern. Fetal Neonatal Med. 2022, 35, 447–456. [Google Scholar] [CrossRef]
- Briana, D.D.; Fotakis, C.; Kontogeorgou, A.; Gavrili, S.; Georgatzi, S.; Zoumpoulakis, P.; Malamitsi-Puchner, A. Early Human-Milk Metabolome in Cases of Intrauterine Growth-Restricted and Macrosomic Infants. JPEN J. Parenter. Enter. Nutr. 2020, 44, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- Karaer, A.; Mumcu, A.; Arda, S.; Tuncay, G.; Dogan, B. Metabolomics analysis of placental tissue obtained from patients with fetal growth restriction. J. Obstet. Gynaecol. Res. 2022, 48, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Powell, K.L.; Carrozzi, A.; Stephens, A.S.; Tasevski, V.; Morris, J.M.; Ashton, A.W.; Dona, A.C. Utility of metabolic profiling of serum in the diagnosis of pregnancy complications. Placenta 2018, 66, 65–73. [Google Scholar] [CrossRef]
- Clinton, C.M.; Bain, J.R.; Muehlbauer, M.J.; Li, Y.Y.; Li, L.P.; O’Neal, S.K.; Hughes, B.L.; Cantonwine, D.E.; McElrath, T.F.; Ferguson, K.K. Non-targeted urinary metabolomics in pregnancy and associations with fetal growth restriction. Sci. Rep. 2020, 10, 5307. [Google Scholar] [CrossRef]
- Sovio, U.; Goulding, N.; McBride, N.; Cook, E.; Gaccioli, F.; Charnock-Jones, D.S.; Lawlor, D.A.; Smith, G.C.S. A maternal serum metabolite ratio predicts fetal growth restriction at term. Nat. Med. 2020, 26, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Delplancke, T.D.J.; de Seymour, J.V.; Tong, C.; Sulek, K.; Xia, Y.Y.; Zhang, H.; Han, T.L.; Baker, P.N. Analysis of sequential hair segments reflects changes in the metabolome across the trimesters of pregnancy. Sci. Rep. 2018, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Lee, S.M.; Byun, D.J.; Kim, S.Y.; Kim, H.I.; Lee, D.Y.; Jung, Y.M.; Park, C.W.; Park, J.S.; Choi, M.H. Maternal Signatures of Cortisol in First Trimester Small-for-Gestational Age. Reprod. Sci. 2022, 29, 1498–1505. [Google Scholar] [CrossRef]
- Morillon, A.C.; Leite, D.F.B.; Yakkundi, S.; Gethings, L.A.; Thomas, G.; Baker, P.N.; Kenny, L.C.; English, J.A.; McCarthy, F.P. Glycerophospholipid and detoxification pathways associated with small for gestation age pathophysiology: Discovery metabolomics analysis in the SCOPE cohort. Metabolomics 2021, 17, 5. [Google Scholar] [CrossRef]
- Alexandre-Gouabau, M.C.; Courant, F.; Le Gall, G.; Moyon, T.; Darmaun, D.; Parnet, P.; Coupe, B.; Antignac, J.P. Offspring Metabolomic Response to Maternal Protein Restriction in a Rat Model of Intrauterine Growth Restriction (IUGR). J. Proteome Res. 2011, 10, 3292–3302. [Google Scholar] [CrossRef]
- Chang, E.I.; Wesolowski, S.R.; Gilje, E.A.; Baker, P.R.; Reisz, J.A.; D’Alessandro, A.; Hay, W.W.; Rozance, P.J.; Brown, L.D. Skeletal muscle amino acid uptake is lower and alanine production is greater in late gestation intrauterine growth-restricted fetal sheep hindlimb. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R615–R629. [Google Scholar] [CrossRef]
- Huang, S.M.; Li, N.; Liu, C.; Li, T.T.; Wang, W.; Jiang, L.L.; Li, Z.; Han, D.D.; Tao, S.Y.; Wang, J.J. Characteristics of the gut microbiota colonization, inflammatory profile, and plasma metabolome in intrauterine growth restricted piglets during the first 12 hours after birth. J. Microbiol. 2019, 57, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Liu, C.; Feng, C.; Fan, Z.; Dai, Z.; Lai, C.; Li, Z.; Wu, G.; Wang, J. Metabolomic analysis reveals differences in umbilical vein plasma metabolites between normal and growth-restricted fetal pigs during late gestation. J. Nutr. 2012, 142, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Muroya, S.; Zhang, Y.; Kinoshita, A.; Otomaru, K.; Oshima, K.; Gotoh, Y.; Oshima, I.; Sano, M.; Roh, S.; Oe, M.; et al. Maternal Undernutrition during Pregnancy Alters Amino Acid Metabolism and Gene Expression Associated with Energy Metabolism and Angiogenesis in Fetal Calf Muscle. Metabolites 2021, 11, 582. [Google Scholar] [CrossRef] [PubMed]
- Muroya, S.; Zhang, Y.; Otomaru, K.; Oshima, K.; Oshima, I.; Sano, M.; Roh, S.; Ojima, K.; Gotoh, T. Maternal Nutrient Restriction Disrupts Gene Expression and Metabolites Associated with Urea Cycle, Steroid Synthesis, Glucose Homeostasis, and Glucuronidation in Fetal Calf Liver. Metabolites 2022, 12, 203. [Google Scholar] [CrossRef]
- Nissen, P.M.; Nebel, C.; Oksbjerg, N.; Bertram, H.C. Metabolomics Reveals Relationship between Plasma Inositols and Birth Weight: Possible Markers for Fetal Programming of Type 2 Diabetes. J. Biomed. Biotechnol. 2011, 2011, 378268. [Google Scholar] [CrossRef]
- Wang, Q.E.; Yue, J.; Zhou, X.; Zheng, M.H.; Cao, B.; Li, J. Ouabain regulates kidney metabolic profiling in rat offspring of intrauterine growth restriction induced by low-protein diet. Life Sci. 2020, 259, 118281. [Google Scholar] [CrossRef]
- Kim, H. Glutamine as an immunonutrient. Yonsei Med. J. 2011, 52, 892–897. [Google Scholar] [CrossRef]
- Wu, X.; Xie, C.; Zhang, Y.; Fan, Z.; Yin, Y.; Blachier, F. Glutamate-glutamine cycle and exchange in the placenta-fetus unit during late pregnancy. Amino Acids 2015, 47, 45–53. [Google Scholar] [CrossRef]
- Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J. Nutr. 2007, 137, 1539S–1547S; discussion 1548S. [Google Scholar] [CrossRef]
- Saibi, W.; Feki, K.; Yacoubi, I.; Brini, F. Bridging Between Proline Structure, Functions, Metabolism, and Involvement in Organism Physiology. Appl. Biochem. Biotechnol. 2015, 176, 2107–2119. [Google Scholar] [CrossRef]
- Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int. J. Mol. Sci. 2018, 19, 954. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel metabolic and physiological functions of branched chain amino acids: A review. J. Anim. Sci. Biotechnol. 2017, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Kim, J.; Ko, J.W.; Choi, A.; Kwon, Y.H. Effects of maternal branched-chain amino acid and alanine supplementation on growth and biomarkers of protein metabolism in dams fed a low-protein diet and their offspring. Amino Acids 2022, 54, 977–988. [Google Scholar] [CrossRef]
- Litwack, G. Chapter 8—Glycolysis and Gluconeogenesis. In Human Biochemistry; Litwack, G., Ed.; Academic Press: Boston, MA, USA, 2018; pp. 183–198. [Google Scholar]
- Razak, M.A.; Begum, P.S.; Viswanath, B.; Rajagopal, S. Multifarious Beneficial Effect of Nonessential Amino Acid, Glycine: A Review. Oxidative Med. Cell. Longev. 2017, 2017, 1716701. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.; Shine, A.; Hewage, C.; Malthouse, J.P.; Brindle, K.M.; McClenaghan, N.; Flatt, P.R.; Newsholme, P. A nuclear magnetic resonance-based demonstration of substantial oxidative L-alanine metabolism and L-alanine-enhanced glucose metabolism in a clonal pancreatic beta-cell line: Metabolism of L-alanine is important to the regulation of insulin secretion. Diabetes 2002, 51, 1714–1721. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Takahashi, H.; Murao, N.; Gheni, G.; Yokoi, N.; Hamamoto, Y.; Asahara, S.I.; Seino, Y.; Kido, Y.; Seino, S. Glutamate is an essential mediator in glutamine-amplified insulin secretion. J. Diabetes Investig. 2021, 12, 920–930. [Google Scholar] [CrossRef]
- Fowden, A.L. Insulin deficiency: Effects on fetal growth and development. J. Paediatr. Child Health 1993, 29, 6–11. [Google Scholar] [CrossRef]
- Sferruzzi-Perri, A.N.; Owens, J.A.; Pringle, K.G.; Roberts, C.T. The neglected role of insulin-like growth factors in the maternal circulation regulating fetal growth. J. Physiol. 2011, 589, 7–20. [Google Scholar] [CrossRef]
- Eleftheriades, M.; Creatsas, G.; Nicolaides, K. Fetal growth restriction and postnatal development. Ann. N. Y. Acad. Sci. 2006, 1092, 319–330. [Google Scholar] [CrossRef]
- Berends, L.M.; Dearden, L.; Tung, Y.C.L.; Voshol, P.; Fernandez-Twinn, D.S.; Ozanne, S.E. Programming of central and peripheral insulin resistance by low birthweight and postnatal catch-up growth in male mice. Diabetologia 2018, 61, 2225–2234. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Xu, Z.; Cao, J.; Shao, P.; Zhou, M.; Qin, Z.; Liu, Y.; Yu, F.; Zhou, X.; Ji, W.; et al. Proteomics analysis of human placenta reveals glutathione metabolism dysfunction as the underlying pathogenesis for preeclampsia. Biochim. Biophys. Acta. Proteins Proteom. 2017, 1865, 1207–1214. [Google Scholar] [CrossRef]
- De Pace, V.; Chiossi, G.; Facchinetti, F. Clinical use of nitric oxide donors and L-arginine in obstetrics. J. Matern. Fetal Neonatal Med. 2007, 20, 569–579. [Google Scholar] [CrossRef] [PubMed]
- van Eijk, H.M.; Luiking, Y.C.; Deutz, N.E. Methods using stable isotopes to measure nitric oxide (NO) synthesis in the L-arginine/NO pathway in health and disease. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 851, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Duggleby, S.L.; Jackson, A.A. Protein, amino acid and nitrogen metabolism during pregnancy: How might the mother meet the needs of her fetus? Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Suzuki, M.; Saito, T.; Miyado, K. Emerging Role of TCA Cycle-Related Enzymes in Human Diseases. Int. J. Mol. Sci. 2021, 22, 13057. [Google Scholar] [CrossRef]
- Ma, W.; Song, J.; Wang, H.; Shi, F.; Zhou, N.; Jiang, J.; Xu, Y.; Zhang, L.; Yang, L.; Zhou, M. Chronic paradoxical sleep deprivation-induced depression-like behavior, energy metabolism and microbial changes in rats. Life Sci. 2019, 225, 88–97. [Google Scholar] [CrossRef]
- Souza, R.T.; Mayrink, J.; Leite, D.F.; Costa, M.L.; Calderon, I.M.; Rocha Filho, E.A.; Vettorazzi, J.; Feitosa, F.E.; Cecatti, J.G. Metabolomics applied to maternal and perinatal health: A review of new frontiers with a translation potential. Clinics 2019, 74, e894. [Google Scholar] [CrossRef]



| Ref | Case Sample Size | Control Sample Size | Diagnostic Criteria | Bio-Specimen | Sampling Time | Analytic Platform | Targeted/ Untargeted | Upregulated | Downregulated |
|---|---|---|---|---|---|---|---|---|---|
| Georgios Moros (2021) [19] | 41 | 36 | BW < 10th customized centile and abnormal umbilical artery RI | Umbilical cord serum | Not mentioned | 1H NMR | Untargeted | Alanine; Leucine; Isoleucine; Valine | Phenylalanine; Glycerol; Tryptophan |
| Lina Youssef (2021) [20] | 43 | 86 | EFW and BW < 10th centile and abnormal cerebroplacental ratio or abnormal uterine artery pulsatility index, or BW < 3rd centile | Umbilical cord plasma | Immediately after delivery | 1H NMR | Untargeted | Triglycerides (IDL); Cholesterol (IDL) | Cholesterol (HDL); Isoleucine |
| Donata Favretto (2012) [21] | 22 | 21 | EFW < 10th percentile | Umbilical cord serum | Immediately after delivery | LC-HRMS | Untargeted | Valine; Isoleucine; Glutamate; Methionine; Dopamine; Histidine; Proline; Phenylalanine; Uric acid; Caffeine; 5-Methyl-2-undecenoic acid; Tryptophan; Kynurenine; Leu pro; Thyronine; Oleic acid; Hexadecanedioic acid; Phe phe; Arg cys asn; Arg phe arg; Trp arg arg; 1-Hydroxyvitamin D3 3-dglucopyranoside | None |
| Ray Oliver Bahado-Singh (2019) [22] | 39 | 39 | BW < 10th percentile | Umbilical cord serum | Within 20 min of delivery | LC-MS/MS 1H NMR | Untargeted | Lysine; Threonine; Dopa; Kynurenine; LysoPC a C18:1; LysoPC a C20:3; PC aa C36:1; PC aa C36:3; PC aa C38:3; PC ae C44:5; Arginine | Creatinine; C0; C10:1; C12:1; C16:1; C2; C4; C4-OH; PC aa C24.0; PC aa C26.0; PC aa C28:1; PC aa C30:0; PC aa C32:0; PC aa C32:1; PC aa C34:2; PC aa C36:4; PC aa C36:6; PC aa C38:4; PC aa C38:5; PC aa C40:4; PC aa C40:6; PC aa C42:2; PC aa C42:4; PC aa C42:5; PC aa C42:6; PC ae C30:2; PC ae C36:0; PC ae C36:3; PC ae C36:5; PC ae C38:4; PC ae C38:6; PC ae C42:1; 2-Hydroxybutyrate; 3-Hydroxybutyrate; Acetate; Acetoacetate; Choline; Creatine; Formate |
| Jezid Miranda (2018) [23] | 27 | 28 | BW < 3rd centile and/or abnormal uterine artery doppler and/or abnormal cerebroplacental ratio | Umbilical cord plasma | Immediately after delivery | 1H NMR | Untargeted +targeted | Cholesterol (VLDL); Triglycerides (VLDL); Triglycerides (IDL); Acetate; Formate | None |
| Magdalena Sanz-Cortés (2013) [24] | 20 | 23 | BW < 10th centile and abnormal umbilical artery | Umbilical cord serum | At delivery | 1H NMR | Untargeted | Triglycerides; Creatine; Glutamine (Early-onset FGR) | Glucose; Choline; Phenylalanine (Early-onset FGR) Choline; Glutamine; Tyrosine; Valine; Leucine; Alanine (Late-onset FGR) |
| Carmen Ivorra (2012) [25] | 20 | 30 | BW < 10th percentile | Umbilical cord plasma | Immediately after delivery | NMR | Untargeted | Citrulline; Phenylalanine | Proline; Choline; Glutamine; Alanine; Glucose |
| Aviv Schupper (2021) [26] | 6380 | 61,068 | BW < 10th percentile | Neonatal dried blood | Between 36 and 72 h from birth | HPLC-MS | Targeted | Alanine; Methionine; Proline; Carnitine | Valine |
| Angelica Dessı` (2014) [27] | 12 | 17 | Ultrasonographically in the prenatal period and BW < 10th percentile | Neonatal urine | Within 8 h of delivery | 1H NMR | Untargeted | Citrate; Creatinine; Creatine; Myo-inositol; Betaine/Trimethylamine N-oxide; Glycine | None |
| Juan Manuel Chao de la Barca (2022) [28] | 15 | 15 | BW < 10th percentile and doppler abnormalities | Umbilical cord plasma | Not mentioned | LC-MS/MS FIA-MS/MS | Targeted | C0; C4; C2; Alanine; Asparagine; Tyrosine; Glutamine; Proline; Alpha-aminoadipic acid; Trans-4-hydroxyproline; Spermine; LysoPC a C26:1; PC aa C32:0; PC aa C24:0 | LysoPC a C18:0; LysoPC a C17:0; LysoPC a C18:1; LysoPC a C20:3; LysoPC a C18:2; LysoPC a C20:4; LysoPC a C16:1; LysoPC a C16:0; PC ae C40:6; PC ae C42:5; PC aa C38:6; PC aa C36:0; PC aa C42:0; PC aa C42:6; PC ae C42:4; PC ae C40:2; PC ae C38:6; PC aa C36:1; PC ae C40:3; PC ae C40:5; PC ae C40:4; PC aa C36:3; PC ae C42:2; PC ae C40:1; PC aa C40:6; PC ae C42:3; PC ae C44:5; PC aa C38:0; PC ae C38:0; PC aa C36:6; PC aa C38:3; PC ae C44:4; PC ae C38:3; PC ae C44:6; PC ae C36:3; SM(OH)C22:1; SM(OH)C24:1; SM C24:0; SM C26:0; Tryptophan |
| Ref | Case Sample Size | Control Sample Size | Diagnostic Criteria | Bio-Specimen | Sampling Time | Analytic Platform | Targeted/ Untargeted | Upregulated | Downregulated |
|---|---|---|---|---|---|---|---|---|---|
| Georgios Moros (2021) [19] | 41 | 36 | BW < 10th customized centile and abnormal umbilical artery RI | Maternal serum | During delivery | 1H NMR | Untargeted | Alanine; Leucine; Isoleucine; Valine | Phenylalanine; Glycerol; 3-Hydroxybutyrate |
| Chelsea M. Clinton (2020) [35] | 30 | 30 | BW < 10th percentile | Urine | 10 w | GC-MS | Untargeted | Benzoic acid; Malonic acid; 2-Ketoleucine/ketoisoleucine; 2-Ketobutyric acid; 2-Methylglutaric acid; Acetoacetate | None |
| 26 w | 1,2-Propanediol; Kynurenic acid; N-heptanoic acid; Benzoic acid | None | |||||||
| Léa Maitre (2014) [29] | 36 | 275 | BW < 10th percentile of predicted BW distribution | Urine | 11–13 w | 1H NMR | Untargeted | None | Tyrosine; Lactate; Alanine; Acetate; Citrate; Trimethylamine; Glycine; Formate |
| Ulla Sovio (2020) [36] | 175 | 299 | BW < 3rd percentile, or BW between the 3rd and 10th percentile and the lowest decile of fetal abdominal growth velocity | Maternal serum | 20/28/36 w | UPLC-MS/MS | Untargeted | 1-(1-Enyl-stearoyl)2-oleoyl-GPC (P-18:0/18:1); 1,5-Anhydroglucitol; Cotinine N-oxide; 4-Androsten-3beta,17beta-diol monosulfate; Hydroxycotinine; Acisoga; 3-Hydroxycotinine glucuronide; O-cresol sulfate; Dehydroisoandrosterone sulfate | 5Alpha-androstan-3Alpha,17alpha-diol disulfate; Estriol 3-sulfate; 4-Cholesten-3-one; Pregnanolone/allopregnanolone sulfate; 5Alpha-pregnan-3alpha,20beta-diol disulfate 1; N1,N12-diacetylspermine; 17Alpha-hydroxypregnanolone glucuronide; 5Alpha-pregnan-3beta,20beta-diol monosulfate; Progesterone; Pregnanediol-3-glucuronide |
| Karolina Sulek (2014) [30] | 41 | 42 | BW < 10th corrected birth centile | Maternal hair | 26–28 w | GC-MS | Untargeted | Palmitate; 2-Methyloctadecanoate; Myristate; Margarate; Stearate; Dodecanoate; Octanoate; Heptadecane; Nicotinamide | 3-Hydroxybenzoate; Levulinic acid; 1-Aminocyclopropane-1-carboxylate; Citraconate; Lactate; Glycine; Proline; Isoleucine; Serine; Leucine; Glutamate; Phenylalanine; Alanine; Valine; Aspartate; Threonine; Tyrosine; Methionine; Lysine; Pyroglutamate; Ornithine; Glutathione |
| Jezid Miranda (2018) [23] | 27 | 28 | BW < 3rd centile and/or abnormal uterine artery doppler and/or abnormal cerebroplacental ratio | Maternal plasma | 2–4 h after delivery | 1H NMR | Untargeted + targeted | None | Triglycerides (HDL); Alanine; Citrate; 2-oxoisovaleric acid; Pyruvate |
| Ray Oliver Bahado-Singh (2020) [31] | 19 | 30 | BW < 10th percentile | Placenta | Within 20 min of delivery | DI-LC-MS/MS 1H NMR | Untargeted | Citrate | 3-Hydroxybutyrate; Glycine; D-Glucose; t4-OH-Pro; Symmetric dimethylarginine; Sarcosine; Kynurenine; Methionine sulfoxide; Alanine; Aspartate; Spermidine; Valine; PC ae C424; Myo-inositol |
| Despina D. Briana (2020) [32] | 19 | 60 | BW ≤ 10th customized percentile | Human-milk | Within 3–4 d of delivery | 1H NMR | Untargeted | Citrate; Choline; Lactose; N-acetylglutamine; Phosphocoline | Isoleucine; Valine |
| Thibaut D. J. Delplancke (2018) [37] | 20 | 73 | BW < 10th customized centile | Hair | In the second trimester | GC-MS LC-MS | Untargeted | Myristic acid; Pentadecanoic acid; Margaric acid | None |
| Abdullah Karaer (2022) [33] | 10 | 14 | AC/EFW ratio < 3rd percentile | Placenta | Within 5 min of delivery | 1H HR-MAS NMR | Untargeted | Lactate; Glutamine; Glycerophosphocholine; Phosphocholine; Taurine; Myo-inositol | None |
| Chaelin Lee (2022) [38] | 56 | 56 | BW < 10th percentile | Maternal serum | 10–14 w | LC-MS | Targeted | Tetrahydrocortisol | 21-Deoxycortisol |
| Aude-Claire Morillon (2021) [39] | 40 | 40 | BW < 10th percentile | Urine | 20 w | UPLC-MS | Untargeted | None | Sulfolithocholic acid; Estriol-16-glucuronide; 4-Hydroxybenzaldehyde; Neuromedin N; D-Glucuronic acid; 18-Hydroxycortisol; Beta-1,4-Mannosyl-N-acetylglucosamine |
| 40 | 40 | Maternal serum | PE(P-31:0); PE(42:1); PE(36:4); PS(O-37:0); PS(41:5); PS(37:2); PS(43:6); PS(P-34:0); PC(O-42:4); PC(40:5); PC(38:6); LysoPC(16:0); PA(O-36:2); LysoPA(18:1); PI(37:1); PI(P-33:1); PGP(38:4); PGP(40:4); PG(36:6); PG(39:8); PG(38:4); DG(44:4); DG(O-34:1); N,N-dimethyl arachidonoyl amine; N-palmitoyl valine; SM(34:1) Ganglioside GA2 (40:1); Cer(34:0); Cer(39:2) | CL(72:2); TG(64:15); 8S-hydroxy-hexadecanoic acid; CE(17:0) | |||||
| Katie L. Powel (2018) [34] | 34 | 82 | EFW < 10th percentile and abnormal placental vascular resistance | Maternal serum | 26–41 w | 1H NMR | Untargeted | Glutamate; Glutamine | 3-Hydroxybutyrate |
| Juan Manuel Chao de la Barca (2022) [28] | 20 + 24 | 20 + 22 | BW < 10th percentile and doppler abnormalities | Placenta | Within 30 min after delivery | LC-MS/MS FIA-MS/MS | Targeted | C0; C2; C4; C4-OH; C16; C18; C18:1; C5; C16:1; C18:2; Tryptophan; Creatinine; PC ae C36:5; Hexose | Aspartate; Threonine; Kynurenine; Putrescine; Spermidine; Trans-4-hydroxyproline; LysoPC a C18:0; LysoPC a C20:3; LysoPC a C20:4; PC aa C36:1; PC aa C38:0; PC aa C40:3; PC ae C42:3; SM C24:0 SM C26:0; SM C24:1; SM (OH) C22:1; Glycine; Serine; Arginine; Tyrosine; Alpha-aminoadipic acid; LysoPC a C16; LysoPC a C16:1; LysoPC a C17:0; LysoPC a C18:1; LysoPC a C18:2; PC aa C32:0; PC ae C34:0; PC ae C40:2; PC ae C44:5; SM C26:1; SM(OH) C22:2; SM(OH) C24:1; Methionine; Carnosine; LysoPC a C24:0; LysoPC a C28:1; PC aa C36:3; PC aa C38:3; PC aa C42:0; PC ae C30:0; PC ae C30:2; PC ae C36:1; PC ae C36:2; PC ae C36:3; PC ae C38:2; PC ae C38:3; PC ae C40:3; PC ae C40:4; PC ae C42:4; PC ae C42:6; SM C16:1; SM(OH) C14:1 |
| Ref | Bio-Specimen | Potential Biomarkers | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|
| Georgios Moros (2021) [19] | Maternal and umbilical cord blood | Alanine | - | - | 0.871 (Umbilical cord blood) 0.792 (Maternal blood) |
| Isoleucine | - | - | 0.795 (Umbilical cord blood) 0.812 (Maternal blood) | ||
| Leucine | - | - | 0.816 (Umbilical cord blood) 0.773 (Maternal blood) | ||
| Valine | - | - | 0.785 (Umbilical cord blood) 0.786 (Maternal blood) | ||
| Phenylalanine | - | - | 0.779 (Umbilical cord blood) 0.75 (Maternal blood) | ||
| Glycerol | - | - | 0.853 (Umbilical cord blood) 0.751 (Maternal blood) | ||
| Tryptophan | - | - | 0.751 (Umbilical cord blood) | ||
| 3-Hydroxybutyrate | - | - | 0.774 (Maternal blood) | ||
| Ulla Sovio (2020) [36] | Maternal serum | (A)1-(1-Enyl-stearoyl)-2-oleoyl-GPC (P-18:0/18:1) | - | - | 0.640 |
| (B)1,5-Anhydroglucitol | - | - | 0.650 | ||
| (C)5α-Androstan-3α,17α-diol disulfate | - | - | 0.690 | ||
| (D)N1,N12-diacetylspermine | - | - | 0.660 | ||
| A × B | - | - | 0.700 | ||
| C × D | - | - | 0.710 | ||
| (A × B)/ (C × D) | - | - | 0.780 | ||
| Karolina Sulek (2014) [30] | Maternal hair | Lactate + levulinate + 2-methyloctadecanate + tyrosine + margarate | - | - | 0.998 |
| Ray O. Bahado-Singh (2020) [31] | Placenta | 3-Hydroxybutyric acid + glycine + PC aa C420 | 0.867 | 0.842 | 0.912 |
| LysoPC a C261 + PC ae C382 + PC ae C446 | 0.867 | 0.737 | 0.796 | ||
| 3-Hydroxybutyric acid + glycine + PC aa C420 + medical disorder + maternal age + prior FGR + race + gravidity | 0.833 | 0.789 | 0.832 | ||
| Ray Oliver Bahado-Singh (2019) [22] | Cord blood serum | Creatinine + C14 + C2 + C4 + LysoPC.a.C16:1 + LysoPC.a.C18:1 + LysoPC.a.C20:3 + LysoPC.a.C20:4 + LysoPC.a.C28:1 + PC.aa.C24:0 + PC.aa.C36:4 + PC.aa.C38:4 + PC.aa.C42:4 + EDTAca_N + creatine | 0.870 | 0.830 | 0.910 |
| LysoPC.a.C16:1 + C2 + creatinine + LysoPC.a.C18:2 + LysoPC.a.C18:1 + LysoPC.a.C20:3 + PC.aa.C24:0 + C6.C4:1.DC. + C4 + C10:1 + C16:1 + C12:1 + C12 + C0 + LysoPC.a.C28:1 | 0.830 | 0.850 | 0.870 | ||
| Creatinine + LysoPC.a.C16:1 + LysoPC.a.C20:3 + C2 + LysoPC.a.C18:2 + C4 + C12:1 + EDTAca_N + C6.C4:1.DC. + taurine + C16:2 + C0 + putrescine + PC.aa.C24:0 + LysoPC.a.C28:1 | 0.850 | 0.790 | 0.880 | ||
| Creatinine + C2 + C4 + LysoPC.a.C16:1 + LysoPC.a.C2:.3 + LysoPC.a.C28:1 + PC.aa.C24:0 | 0.830 | 0.870 | 0.880 | ||
| Creatinine + C2 + C4 + LysoPC.a.C16:1 + LysoPC.a.C2:.3 + LysoPC.a.C28:1 + PC.aa.C24:0 + prior FGR + maternal age + gravity + race | 0.700 | 0.730 | 0.690 | ||
| Chaelin Lee (2022) [38] | Maternal serum | 21-DeoxyF + F/21-deoxyF(cortisol to 21-deoxycortisol) + THF/F(tetrahydrocortisol to cortisol) | 0.734 | 0.817 | 0.824 |
| Katie L. Powel (2018) [34] | Placenta | 3-Hydroxybutyrate | 0.939 0.364 | 0.300 0.545 | 0.623 (Discovery) 0.581 (Validation) |
| Ref | Case Sample Size | Control Sample Size | FGR Model | Bio-Specimen | Sampling Time | Analytic Platform | Targeted/ Untargeted | Upregulated | Downregulated |
|---|---|---|---|---|---|---|---|---|---|
| Alexandre-Gouabau (2011) [40] | 4 | 10 | Fetal rats of low protein diet maternal rats | Fetal plasma | Postnatal 0 d | LC-HRMS | Untargeted | None | Proline; Arginine; Histidine |
| Eileen I. Chang (2019) [41] | 10 | 8 | Fetal sheep of placental insufficiency | Fetal skeletal muscle | Late gestation | HPLC | Untargeted | 2-Oxo-7-methylthioheptanoic acid; 2-Hydroxyglutarate; N-acetylneuraminate | Cystathionine; N-acetylcitrulline; Gamma-glutamylalanine; N-carbamyl-L-glutamate; Gamma-glutamylcysteine; Arginine; Aspartate; N,N-dimethylglycine; Adenosine; 5-Hydroxylysine; 4-Hydroxyproline |
| Fetal arterial and venous plasma | 4-Pyridoxate; 2-Aminoadipate; Triacanthine; Dopamine; Uric Acid; Citrate; Adrenaline; Carnitine; 5,6-Dihydrothymine; Arabitol; Guanine; Taurine; Dehydroascorbate; Alanine | Orthophosphate; Diphosphate; 3D-(3,5/4)-trihydroxycyclohexane-1,2-dione; Fructose 1,6-bisphosphate; 5-Hydroxylysine; 2-Methyleneglutarate; N-Acyl-D-mannosaminolactone; Pyruvate; 1,4-Beta-D-xylan; Glucose; Cys gly; Ascorbate; Arginine; Sn-glycerol 3-phosphate; Succinate; Ribose | |||||||
| Shimeng Huang (2019) [42] | 6 | 6 | Piglets with a birth weight 2 sd below the mean | Fetal plasma | Immediately after birth | UPLC-MS | Untargeted | 6’-Sialyllactose; Faradiol; Neotigogenin; Zymosterol intermediate 2, 13, 14-Dihydro PGF-1 alpha; 6, 10, 14-Trimethyl-5, 9, 13-pentadecatrien-2-one | Serotonin; Dihydrowyerone |
| Gang Lin (2012) [43] | 9 | 9 | Piglets with a birth weight 2 sd below the mean | Umbilical vein plasma | 90 d of gestation | HPLC-Q-TOFMS | Untargeted | Pyroglutamic acid | Proline; Valine; Phenylalanine; Isoleucine; Leucine; Tyrosine; Glutamine; Arginine; Dodecanoylcarnitine |
| 9 | 9 | 110 d of gestation | Creatinine; Carnitine; Pyroglutamic acid | Tryptophan; Glutamine; Arginine; Dodecanoylcarnitine | |||||
| Susumu Muroya (2021) [44] | 4 | 4 | Calves of maternal undernutrition | Fetal muscle | 260 ± 8.3 d of gestation | CE-TOFMS | Untargeted | Carnosine; Glutamine; Glycerol; Creatine; N6-methyllysine; Phosphorylcholine; Phenylalanine; Proline | Myo-inositol 2-phosphate; 2-Aminoethylphosphonic acid |
| Susumu Muroya (2022) [45] | 4 | 4 | Calves of maternal undernutrition | Fetal liver | 260 ± 8.3 d of gestation | CE-TOFMS | Untargeted | Aspartate; Betaine aldehyde; Glycerol; 3-Aminopropane1,2-diol; Alanine; 6-Phosphogluconate; Ophthalmate | 4-Amino-3-hydroxybutyrate; 2-Aminoethylphosphonate; UDP-glucose/UDP-galactose; N5-Ethylglutamine; UDP-glucuronate; 2-Hydroxybutyrate; Octanoate; Gly leu |
| PiaMarlene Nissen (2011) [46] | 12 | 12 | Piglets with low birth weight | Umbilical cord plasma | 110 d of gestation | GC-MS | Untargeted | Myoinositol; D-chiro-inositol | None |
| Qien Wang (2020) [47] | 53 | 57 | Fetal rats of low protein diet maternal rats | Fetal kidney | 20 d of gestation | GC-MS LC-MS | Untargeted | Xanthine; Xanthosine; L-lysine; Oleate; Arachidonate; Phenylalanine; Glutamine; Guanine | N-acetylornithine; Arginine; GMP; UMP; Acetyl-CoA; Carbamoyl phosphate; dAMP; UDP; TMP; dTDP; Urea; Pantothenate |
| No | Metabolites | Hits | Up | Down |
|---|---|---|---|---|
| 1 | Arginine | 4 | - | Fetal rats plasma [40] Fetal sheep skeletal muscle+ arterial and venous plasma [41] Piglets umbilical vein plasma [43] Fetal rats kidney [47] |
| 2 | Glutamine | 3 | Calves muscle [44] Fetal rats kidney [47] | Piglets umbilical vein plasma [43] |
| 3 | Phenylalanine | 3 | Calves muscle [44] Fetal rats kidney [47] | Piglets umbilical vein plasma [43] |
| 4 | Proline | 3 | Calves muscle [44] | Fetal rats plasma [40] Piglets umbilical vein plasma [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, M.; Yang, Z.; Rong, X.; Hu, X.; Yao, N.; Zhu, M.; Wang, X.; Zhu, X.; Yin, J. The Exploration of Fetal Growth Restriction Based on Metabolomics: A Systematic Review. Metabolites 2022, 12, 860. https://doi.org/10.3390/metabo12090860
Yao M, Yang Z, Rong X, Hu X, Yao N, Zhu M, Wang X, Zhu X, Yin J. The Exploration of Fetal Growth Restriction Based on Metabolomics: A Systematic Review. Metabolites. 2022; 12(9):860. https://doi.org/10.3390/metabo12090860
Chicago/Turabian StyleYao, Mengxin, Zhuoqiao Yang, Xin Rong, Xuan Hu, Na Yao, Manting Zhu, Xinnan Wang, Xiaoyan Zhu, and Jieyun Yin. 2022. "The Exploration of Fetal Growth Restriction Based on Metabolomics: A Systematic Review" Metabolites 12, no. 9: 860. https://doi.org/10.3390/metabo12090860
APA StyleYao, M., Yang, Z., Rong, X., Hu, X., Yao, N., Zhu, M., Wang, X., Zhu, X., & Yin, J. (2022). The Exploration of Fetal Growth Restriction Based on Metabolomics: A Systematic Review. Metabolites, 12(9), 860. https://doi.org/10.3390/metabo12090860