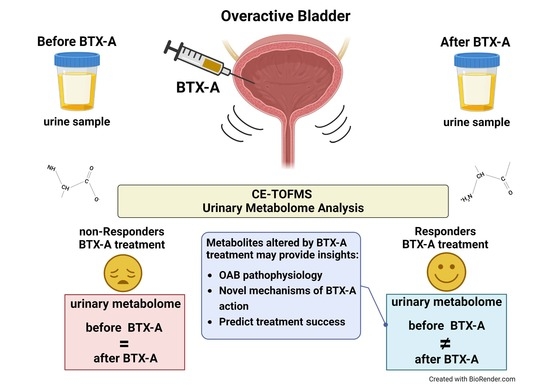
A Feasibility Study to Evaluate Changes in Urinary Metabolites after OnabotulinumtoxinA Injection for Refractory Overactive Bladder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Intravesical BTX-A Injection
2.4. Overactive Bladder Symptom Score (OAB-V8)
2.5. Urinary Metabolome Analysis
2.6. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Metabolites
3.2.1. Adenosine
3.2.2. N8-Acetylspermidine
3.2.3. Guanidinoacetic Acid
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; Wein, A. The standardization of terminology in lower urinary tract function: Report from the standardisation sub-committee of the International Continence Society. Urology 2003, 61, 37–49. [Google Scholar] [CrossRef]
- Hashim, H.; Abrams, P. Is the bladder a reliable witness for predicting detrusor overactivity? J. Urol. 2006, 175, 191–195. [Google Scholar] [CrossRef]
- Digesu, G.A.; Khullar, V.; Cardozo, L.; Salvatore, S. Overactive bladder symptoms: Do we need urodynamics? Neurourol. Urodyn. 2003, 22, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Munoz, A.; Smith, C.P.; Boone, T.B.; Somogyi, G.T. Overactive and underactive bladder dysfunction is reflected by alterations in urothelial ATP and NO release. Neurochem. Int. 2011, 58, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C. Potential urine and serum biomarkers for patients with bladder pain syndrome/interstitial cystitis. Int. J. Urol. 2014, 21 (Suppl. S1), 34–41. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, B.M.; Stirdivant, S.M.; Mitchell, M.W.; Wulff, J.E.; McDunn, J.E.; Li, Z.; Wolfert, R.L. Bladder cancer biomarker discovery using global metabolomic profiling of urine. PLoS ONE 2014, 9, e115870. [Google Scholar] [CrossRef]
- Dettmer, K.; Aronov, P.A.; Hammock, B.D. Mass spectrometry-based metabolomics. Mass Spectrom. Rev. 2007, 26, 51–78. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.I.; Atherton, H.J.; Goodacre, R.; Griffin, J.L. Systems level studies of mammalian metabolomes: The roles of mass spectrometry and nuclear magnetic resonance spectroscopy. Chem. Soc. Rev. 2011, 40, 387–426. [Google Scholar] [CrossRef]
- Mossa, A.H.; Shamout, S.; Cammisotto, P.; Campeau, L. Urinary metabolomics predict the severity of overactive bladder syndrome in an aging female population. Int. Urogynecol. J. 2020, 31, 1023–1031. [Google Scholar] [CrossRef]
- Schurch, B.; Stöhrer, M.; Kramer, G.; Schmid, D.M.; Gaul, G.; Hauri, D. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: A new alternative to anticholinergic drugs? Preliminary results. J. Urol. 2000, 164, 692–697. [Google Scholar] [CrossRef]
- Villacampa, F.; Ruiz, M.A.; Errando, C.; Arlandis, S.; Arumí, D.; Lizarraga, I.; Rejas, J. Predicting self-perceived antimuscarinic therapy effectiveness on overactive bladder symptoms using the Overactive Bladder 8-Question Awareness Tool. Int. Urogynecol. J. 2013, 24, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.C.; Sehgal, A.; Crump, R.T.; Baverstock, R.; Sutherland, J.M.; Carlson, K. Evaluating the 8-item overactive bladder questionnaire (OAB-v8) using item response theory. Neurourol. Urodyn. 2018, 37, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Soga, T.; Heiger, D.N. Amino acid analysis by capillary electrophoresis electrospray ionization mass spectrometry. Anal. Chem. 2000, 72, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Soga, T.; Ueno, Y.; Naraoka, H.; Ohashi, Y.; Tomita, M.; Nishioka, T. Simultaneous determination of anionic intermediates for Bacillus subtilis metabolic pathways by capillary electrophoresis electrospray ionization mass spectrometry. Anal. Chem. 2002, 74, 2233–2239. [Google Scholar] [CrossRef]
- Soga, T. Quantitative metabolome analysis using capillary electrophoresis mass spectrometry. J. Proteome Res. 2003, 2, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics 2010, 6, 78–95. [Google Scholar] [CrossRef]
- Merrill, L.; Gonzalez, E.J.; Girard, B.M.; Vizzard, M.A. Receptors, channels, and signalling in the urothelial sensory system in the bladder. Nat. Rev. Urol. 2016, 13, 193–204. [Google Scholar] [CrossRef]
- Antunes-Lopes, T.; Cruz, F. Urinary Biomarkers in Overactive Bladder: Revisiting the Evidence in 2019. Eur. Urol. Focus 2019, 5, 329–336. [Google Scholar] [CrossRef]
- Mudumba, S.; Menezes, A.; Fries, D.; Blankenship, J. Differentiation of PC12 cells induced by N8-acetylspermidine and by N8-acetylspermidine deacetylase inhibition. Biochem. Pharmacol. 2002, 63, 2011–2018. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H.; Tracey, K.J. Regulation of macrophage activation and inflammation by spermine: A new chapter in an old story. Crit. Care Med. 2000, 28 (Suppl. S4), N60–N66. [Google Scholar] [CrossRef]
- Birder, L.; Andersson, K.E. Urothelial signaling. Physiol. Rev. 2013, 93, 653–680. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, M.E.; Brosnan, J.T. Renal Arginine Metabolism. J. Nutr. 2004, 134, 2791S–2795S. [Google Scholar] [CrossRef] [PubMed]
- Karakus, S.; Musicki, B.; Navati, M.S.; Friedman, J.M.; Davies, K.P.; Burnett, A.L. NO-Releasing Nanoparticles Ameliorate Detrusor Overactivity in Transgenic Sickle Cell Mice via Restored NO/ROCK Signaling. J. Pharmacol. Exp. Ther. 2020, 373, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Shimura, H.; Mitsui, T.; Kira, S.; Ihara, T.; Sawada, N.; Nakagomi, H.; Takeda, M. Metabolomic Analysis of Overactive Bladder in Male Patients: Identification of Potential Metabolite Biomarkers. Urology 2018, 118, 158–163. [Google Scholar] [CrossRef]



| Characteristics | Total n = 21 | No improvement n = 6 | Mild n = 3 | Moderate n = 6 | Marked n = 6 | p Value |
|---|---|---|---|---|---|---|
| Age (y), mean (SD) | 56.8 (10.9) | 55.2 (13.7) | 51.33 (6.7) | 54.83 (9.0) | 63 (10.8) | 0.41 |
| BMI (kg/m2), mean (SD) | 31.3 (4.9) | 29.7 (5.7) | 29.67 (3.5) | 32.83 (4.6) | 32.23 (5.4) | 0.65 |
| Race/Ethnicity, n (%) | 0.23 | |||||
| Black | 4 (19.1) | 0 | 0 | 1 (16.7) | 3 (50.0) | |
| Hispanic | 16 (76.2) | 5 (83.3) | 3 (100) | 5 (83.3) | 3 (50.0) | |
| Other | 1 (4.8) | 1 (16.7) | 0 | 0 | 0 | |
| Diabetes, n (%) | 3 (14.3) | 2 (33.3) | 0 | 1 (16.7) | 0 | 0.35 |
| Smoking history, n (%) | 1 (4.8) | 0 | 0 | 0 | 1 (16.7) | 0.45 |
| Previous pelvic surgery, n (%) | 8 (38.1) | 2 (33.3) | 2 (66.7) | 2 (33.3) | 2 (33.3) | 0.75 |
| Metabolite | Improvement in OAB-V8 Score a | |||
|---|---|---|---|---|
| None [Reference] | Mild ß (95 % CI) § | Moderate ß (95 % CI) § | Marked ß (95 % CI) § | |
| Adenosine | ||||
| Unadjusted model | 27.0 | −127.0 (−251.42, −2.59) * | −127.0 (−239.54, −14.46) * | −37.0 (−132.11, 58.11) |
| Adjusted model b | [Reference] | −123.74 (−229.16, −18.32) * | −106.91 (−206.24, −7.59) * | −23.09 (−120.49, 74.31) |
| N-8 Acetylspermidine | ||||
| Unadjusted model | −19.0 | 10.0 (−86.71, 106.71) | 100.0 (14.71, 185.29) * | 28.0 (−57.29, 113.29) |
| Adjusted model b | [Reference] | 3.03 (−89.81, 95.87) | 124.23 (39.44, 209.02) ** | 56.0 (−35.84, 147.85) |
| Guanidinoacetic acid | ||||
| Unadjusted model | −4.0 | 30.0 (−53.79, 113.79) | 91.0 (10.05, 171.95) * | 71.0 (−9.95, 151.95) |
| Adjusted model b | [Reference] | 15.70 (−91.95, 123.34) | 46.86 (−58.35, 152.09) | 60.51 (−54.43, 175.45) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tellechea, L.M.; Freeman, S.; Agalliu, I.; Laudano, M.A.; Suadicani, S.O.; Abraham, N. A Feasibility Study to Evaluate Changes in Urinary Metabolites after OnabotulinumtoxinA Injection for Refractory Overactive Bladder. Metabolites 2022, 12, 880. https://doi.org/10.3390/metabo12090880
Tellechea LM, Freeman S, Agalliu I, Laudano MA, Suadicani SO, Abraham N. A Feasibility Study to Evaluate Changes in Urinary Metabolites after OnabotulinumtoxinA Injection for Refractory Overactive Bladder. Metabolites. 2022; 12(9):880. https://doi.org/10.3390/metabo12090880
Chicago/Turabian StyleTellechea, Laura M., Samantha Freeman, Ilir Agalliu, Melissa A. Laudano, Sylvia O. Suadicani, and Nitya Abraham. 2022. "A Feasibility Study to Evaluate Changes in Urinary Metabolites after OnabotulinumtoxinA Injection for Refractory Overactive Bladder" Metabolites 12, no. 9: 880. https://doi.org/10.3390/metabo12090880
APA StyleTellechea, L. M., Freeman, S., Agalliu, I., Laudano, M. A., Suadicani, S. O., & Abraham, N. (2022). A Feasibility Study to Evaluate Changes in Urinary Metabolites after OnabotulinumtoxinA Injection for Refractory Overactive Bladder. Metabolites, 12(9), 880. https://doi.org/10.3390/metabo12090880