Solanaceae Glycoalkaloids Disturb Lipid Metabolism in the Tenebrio molitor Beetle
Abstract
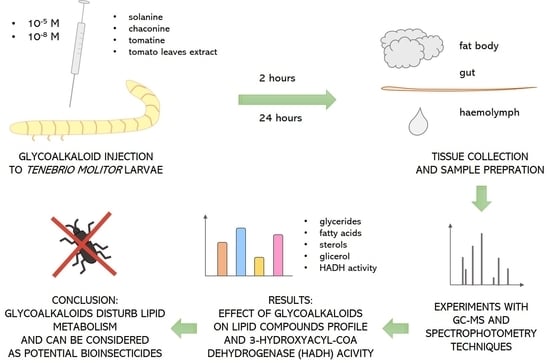
:1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Compounds and Treatment Procedure
2.3. Tissue Isolation
2.4. Lipid Analysis
2.4.1. Sample Preparation
2.4.2. Lipid Level Determination
2.5. Enzyme Activity Analysis
2.5.1. Sample Preparation
2.5.2. Enzyme Activity Measurements
2.6. Statistical Analyses
3. Results
3.1. Level of TAGs
3.2. Changes in Lipid Composition
3.3. Lipid Content and UFA:SFA Ratio
3.4. Enzyme Activity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HADH | 3-hydroxyacyl-CoA dehydrogenase |
| AKH | adipokinetic hormone |
| DAG | diacylglyceride |
| FA | fatty acid |
| GA | glycoalkaloid |
| MDA | malondialdehyde |
| MAG | monoacylglyceride |
| PSM | plant secondary metabolite |
| PUFA | polyunsaturated fatty acid |
| SFA | saturated fatty acid |
| TAG | triacylglyceride |
| UFA | unsaturated fatty acid |
References
- Chapman, R.F. The Insects: Structure and Function; Simpson, S.J., Douglas, A.E., Eds.; Cambridge University Press: New York, NY, USA, 2012. [Google Scholar]
- Canavoso, L.E.; Jouni, Z.E.; Karnas, K.J.; Pennington, J.E.; Wells, M.A. Fat metabolism in insects. Annu. Rev. Nutr. 2001, 21, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.; Liu, S.; Wang, S.; Jiang, R.-J.; Li, S. Hormonal and nutritional regulation of insect fat body development and function. Arch. Insect Biochem. Physiol. 2009, 71, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Schulz, H. Fatty acid oxidation. In Encyclopedia of Biological Chemistry, 2nd ed.; Lennarz, W.J., Lane, M.D., Eds.; Academic Press: Waltham, MA, USA, 2013; pp. 281–284. [Google Scholar]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Chowański, S.; Walkowiak-Nowicka, K.; Winkiel, M.; Marciniak, P.; Urbański, A.; Pacholska-Bogalska, J. Insulin-like peptides and cross-talk with other factors in the regulation of insect metabolism. Front. Physiol. 2021, 12, 973. [Google Scholar] [CrossRef] [PubMed]
- Słocińska, M.; Antos-Krzemińska, N.; Rosiński, G.; Jarmuszkiewicz, W. Nonsulfated sulfakinin changes metabolic parameters of insect fat body mitochondria. Arch. Insect Biochem. Physiol. 2016, 93, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Toprak, U. The role of peptide hormones in insect lipid metabolism. Front. Physiol. 2020, 11, 434. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Plant secondary metabolites modulate insect behavior-steps toward addiction? Front. Physiol. 2018, 9, 364. [Google Scholar] [CrossRef]
- Winkiel, M.J.; Chowański, S.; Słocińska, M. Anticancer activity of glycoalkaloids from Solanum plants: A review. Front. Pharmacol. 2022, 13, 979451. [Google Scholar] [CrossRef]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef]
- Chowański, S.; Adamski, Z.; Marciniak, P.; Rosiński, G.; Buyukguzel, E.; Buyukguzel, K.; Falabella, P.; Scrano, L.; Ventrella, E.; Lelario, F.; et al. A review of bioinsecticidal activity of Solanaceae alkaloids. Toxins 2016, 8, 60. [Google Scholar] [CrossRef]
- Balachandran, C.; Anbalagan, S.; Kandeepan, C.; Arun Nagendran, N.; Jayakumar, M.; Fathi AbdAllah, E.; Alqarawi, A.A.; Hashem, A.; Baskar, K. Molecular docking studies of natural alkaloids as acetylcholinesterase (AChE1) inhibitors in Aedes aegypti. J. Asia Pac. Entomol. 2021, 24, 645–652. [Google Scholar] [CrossRef]
- Marciniak, P.; Kolińska, A.; Spochacz, M.; Chowański, S.; Adamski, Z.; Scrano, L.; Falabella, P.; Bufo, S.A.; Rosiński, G. Differentiated effects of secondary metabolites from Solanaceae and Brassicaceae plant families on the heartbeat of Tenebrio molitor pupae. Toxins 2019, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Ventrella, E.; Marciniak, P.; Adamski, Z.; Rosiński, G.; Chowański, S.; Falabella, P.; Scrano, L.; Bufo, S.A. Cardioactive properties of Solanaceae plant extracts and pure glycoalkaloids on Zophobas atratus. Insect Sci. 2015, 22, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Chowański, S.; Winkiel, M.; Szymczak-Cendlak, M.; Marciniak, P.; Mańczak, D.; Walkowiak-Nowicka, K.; Spochacz, M.; Bufo, S.A.; Scrano, L.; Adamski, Z. Solanaceae glycoalkaloids: α-solanine and α-chaconine modify the cardioinhibitory activity of verapamil. Pharm. Biol. 2022, 60, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Ventrella, E.; Adamski, Z.; Chudzińska, E.; Miadowicz-Kobielska, M.; Marciniak, P.; Buyukguzel, E.; Buyukguzel, K.; Erdem, M.; Falabella, P.; Scrano, L.; et al. Solanum tuberosum and Lycopersicon esculentum leaf extracts and single metabolites affect development and reproduction of Drosophila melanogaster. PLoS ONE 2016, 11, e0155958. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Ortiz, E.V.; Garrido, E.; Poveda, K.; Jander, G. Potato tuber herbivory increases resistance to aboveground lepidopteran herbivores. Oecologia 2016, 182, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Nenaah, G.E. Individual and synergistic toxicity of solanaceous glycoalkaloids against two coleopteran stored-product insects. J. Pest Sci. 2011, 84, 77–86. [Google Scholar] [CrossRef]
- Nenaah, G.E. Toxic and antifeedant activities of potato glycoalkaloids against Trogoderma granarium (Coleoptera: Dermestidae). J. Stored Prod. Res. 2011, 47, 185–190. [Google Scholar] [CrossRef]
- Adamski, Z.; Marciniak, P.; Ziemnicki, K.; Büyükgüzel, E.; Erdem, M.; Büyükgüzel, K.; Ventrella, E.; Falabella, P.; Cristallo, M.; Salvia, R.; et al. Potato leaf extract and its component, α-solanine, exert similar impacts on development and oxidative stress in Galleria Mellonella L. Arch. Insect Biochem. Physiol. 2014, 87, 26–39. [Google Scholar] [CrossRef]
- Spochacz, M.; Chowański, S.; Szymczak, M.; Lelario, F.; Bufo, S.A.; Adamski, Z. Sublethal effects of Solanum nigrum fruit extract and its pure glycoalkaloids on the physiology of Tenebrio molitor (mealworm). Toxins 2018, 10, 504. [Google Scholar] [CrossRef]
- Spochacz, M.; Chowański, S.; Szymczak-Cendlak, M.; Marciniak, P.; Lelario, F.; Salvia, R.; Nardiello, M.; Scieuzo, C.; Scrano, L.; Bufo, S.A.; et al. Solanum nigrum extract and solasonine affected hemolymph metabolites and ultrastructure of the fat body and the midgut in Galleria mellonella. Toxins 2021, 13, 617. [Google Scholar] [CrossRef] [PubMed]
- Yadav, U.C.S. Oxidative stress-induced lipid peroxidation: Role in inflammation. In Free Radicals in Human Health and Disease; Rani, V., Yadav, U.C.S., Eds.; Springer: New Delhi, India, 2015; pp. 119–129. [Google Scholar]
- Büyükgüzel, E.; Büyükgüzel, K.; Erdem, M.; Adamski, Z.; Adamski, Z.; Marciniak, P.; Ziemnicki, K.; Ventrella, E.; Scrano, L.; Bufo, S.A. The influence of dietary α-solanine on the waxmoth Galleria Mellonella L. Arch. Insect Biochem. Physiol. 2013, 83, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Sehnal, F. Compartmentalization of oxidative stress and antioxidant defense in the larval gut of Spodoptera littoralis. Arch. Insect Biochem. Physiol. 2006, 63, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Skowronek, P.; Wójcik, Ł.; Strachecka, A. Fat body—multifunctional insect tissue. Insects 2021, 12, 547. [Google Scholar] [CrossRef] [PubMed]
- Szymczak-Cendlak, M.; Gołębiowski, M.; Chowański, S.; Pacholska-Bogalska, J.; Marciniak, P.; Rosiński, G.; Słocińska, M. Sulfakinins influence lipid composition and insulin-like peptides level in oenocytes of Zophobas atratus beetles. J. Comp. Physiol. B 2022, 192, 15–25. [Google Scholar] [CrossRef]
- Walkowiak-Nowicka, K.; Mirek, J.; Chowański, S.; Sobkowiak, R.; Słocińska, M. Plant secondary metabolites as potential bioinsecticides? Study of the effects of plant-derived volatile organic compounds on the reproduction and behaviour of the pest beetle Tenebrio molitor. Ecotoxicol. Environ. Saf. 2023, 257, 114951. [Google Scholar] [CrossRef]
- Cheung, T.-C.; Cheng, E.C.-C.; Chan, H.-Y.; Tong, S.-K.; Chan, P.-K.; Lee, F.-W.; Wong, Y.-C.; Sin, D.W.-M. Development of a validated database for the authentication of fresh/chilled and frozen pork using β-hydroxylacyl-CoA-dehydrogenases (HADH) assay. Int. J. Food Prop. 2015, 18, 73–80. [Google Scholar] [CrossRef]
- Ravzanaadii, N.; Kim, S.-H.; Choi, W.-H.; Hong, S.-J.; Kim, N.-J. Nutritional value of mealworm, Tenebrio molitor as food source. Int. J. Indust. Entomol. 2012, 25, 93–98. [Google Scholar] [CrossRef]
- Bordiean, A.; Krzyżaniak, M.; Aljewicz, M.; Stolarski, M.J. Influence of different diets on growth and nutritional composition of yellow mealworm. Foods 2022, 11, 3075. [Google Scholar] [CrossRef]
- Kröncke, N.; Neumeister, M.; Benning, R. Near-infrared reflectance spectroscopy for quantitative analysis of fat and fatty acid content in living Tenebrio molitor larvae to detect the influence of substrate on larval composition. Insects 2023, 14, 114. [Google Scholar] [CrossRef]
- Romero-Lorente, M.-Á.; Fabrikov, D.; Montes, J.; Morote, E.; Barroso, F.G.; Vargas-García, M.D.C.; Varga, Á.T.; Sánchez-Muros, M.-J. Pre-treatment of fish by-products to optimize feeding of Tenebrio molitor L. larvae. Insects 2022, 13, 125. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Borgeson, C.E.; Vundla, M. Polyunsaturated fatty acids and eicosanoids in insects. Insect Biochem. 1991, 21, 99–106. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Boguś, M. The metabolism and role of free fatty acids in key physiological processes in insects of medical, veterinary and forensic importance. PeerJ 2021, 9, e12563. [Google Scholar] [CrossRef]
- Stanley-Samuelson, D.W.; Jurenka, R.A.; Cripps, C.; Blomquist, G.J.; de Renobales, I.M. Fatty acids in insects: Composition, metabolism, and biological significance. Arch. Insect Biochem. Physiol. 1988, 9, 1–33. [Google Scholar] [CrossRef]
- Słocińska, M.; Kuczer, M.; Gołębiowski, M. Sulfakinin signalling influences fatty acid levels and composition in Tenebrio molitor beetle. Protein Pept. Lett. 2019, 26, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yu, X.; Feng, Q. Fat body biology in the last decade. Annu. Rev. Entomol. 2019, 64, 315–333. [Google Scholar] [CrossRef]
- Ibarguren, M.; López, D.J.; Escribá, P.V. The effect of natural and synthetic fatty acids on membrane structure, microdomain organization, cellular functions and human health. Biochim. Biophys. Acta 2014, 1838, 1518–1528. [Google Scholar] [CrossRef]
- Vukašinović, E.L.; Pond, D.W.; Grubor-Lajšić, G.; Roger Worland, M.; Kojić, D.; Purać, J.; Popović, Z.D.; Blagojević, D.P. Temperature adaptation of lipids in diapausing Ostrinia nubilalis: An experimental study to distinguish environmental versus endogenous controls. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2018, 188, 27–36. [Google Scholar] [CrossRef]
- Vukašinović, E.L.; Pond, D.W.; Roger Worland, M.; Kojić, D.; Purać, J.; Popović, Z.D.; Grubor-Lajšić, G. Diapause induces remodeling of the fatty acid composition of membrane and storage lipids in overwintering larvae of Ostrinia nubilalis, Hubn. (Lepidoptera: Crambidae). Comp. Biochem. Physiol. B Biochem. 2015, 184, 36–43. [Google Scholar] [CrossRef]
- Friedman, M. Potato glycoalkaloids and metabolites: Roles in the plant and in the diet. J. Agric. Food Chem. 2006, 54, 8655–8681. [Google Scholar] [CrossRef]
- Friedman, M. Tomato glycoalkaloids: Role in the plant and in the diet. J. Agric. Food Chem. 2002, 50, 5751–5780. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; McDonald, G.M.; Filadelfi-Keszi, M. Potato glycoalkaloids: Chemistry, analysis, safety, and plant physiology. Crit. Rev. Plant Sci. 1997, 16, 55–132. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry and anticarcinogenic mechanisms of glycoalkaloids produced by eggplants, potatoes, and tomatoes. J. Agric. Food Chem. 2015, 63, 3323–3337. [Google Scholar] [CrossRef] [PubMed]
- Nepal, B.J.; Stine, K. Glycoalkaloids: Structure, properties, and interactions with model membrane systems. Processes 2019, 7, 513. [Google Scholar] [CrossRef]
- Lunn, J.; Theobald, H.E. The health effects of dietary unsaturated fatty acids. Nutr. Bull. 2006, 31, 178–224. [Google Scholar] [CrossRef]
- Wink, M.; Schimmer, O. Molecular modes of action of defensive secondary metabolites. In Annual Plant Reviews Online; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 21–161. [Google Scholar]
- Sun, H.; Blanford, S.; Guo, Y.; Fu, X.; Shi, W. Toxicity and influences of the alkaloids from Cynanchum komarovii AL. Iljinski (Asclepiadaceae) on growth and cuticle components of Spodoptera litura Fabricius (Noctuidae) larvae. Nat. Prod. Res 2012, 26, 903–912. [Google Scholar] [CrossRef]
- Rharrabe, K.; Jbilou, R.; Bouayad, N.; Ajaha, A.; Aarab, A. Harmaline ingestion effect on development, metabolites and midgut of the red flour beetle, Tribolium castaneum. J. Asia Pac. Entomol. 2020, 23, 29–35. [Google Scholar] [CrossRef]
- Human, H. Resistance of developing honeybee larvae during chronic exposure to dietary nicotine. J. Insect Physiol. 2014, 69, 74–79. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winkiel, M.J.; Chowański, S.; Gołębiowski, M.; Bufo, S.A.; Słocińska, M. Solanaceae Glycoalkaloids Disturb Lipid Metabolism in the Tenebrio molitor Beetle. Metabolites 2023, 13, 1179. https://doi.org/10.3390/metabo13121179
Winkiel MJ, Chowański S, Gołębiowski M, Bufo SA, Słocińska M. Solanaceae Glycoalkaloids Disturb Lipid Metabolism in the Tenebrio molitor Beetle. Metabolites. 2023; 13(12):1179. https://doi.org/10.3390/metabo13121179
Chicago/Turabian StyleWinkiel, Magdalena Joanna, Szymon Chowański, Marek Gołębiowski, Sabino Aurelio Bufo, and Małgorzata Słocińska. 2023. "Solanaceae Glycoalkaloids Disturb Lipid Metabolism in the Tenebrio molitor Beetle" Metabolites 13, no. 12: 1179. https://doi.org/10.3390/metabo13121179
APA StyleWinkiel, M. J., Chowański, S., Gołębiowski, M., Bufo, S. A., & Słocińska, M. (2023). Solanaceae Glycoalkaloids Disturb Lipid Metabolism in the Tenebrio molitor Beetle. Metabolites, 13(12), 1179. https://doi.org/10.3390/metabo13121179