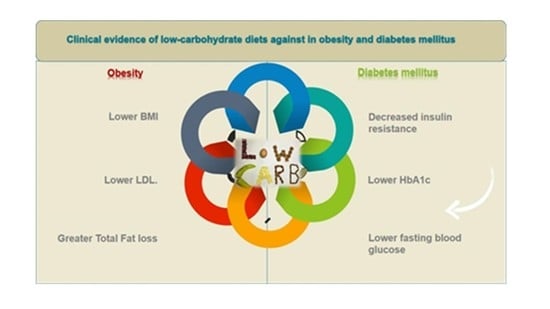
Clinical Evidence of Low-Carbohydrate Diets against Obesity and Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Selection Criteria
3. Results
3.1. Characteristics of the Study
3.2. Results of the Diets
3.2.1. Weight Loss, BMI, Lipids
3.2.2. HbA1c, Blood Glucose, Serum Insulin, Insulin Resistance, Apolipoproteins, and Blood Pressure
4. Discussion
Side Effects of Low Carbohydrate Diets
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Malone, J.I.; Hansen, B.C. Does Obesity Cause Type 2 Diabetes Mellitus (T2DM)? Or Is It the Opposite? Pediatr. Diabetes 2018, 20, 5–9. [Google Scholar] [CrossRef]
- Yao, M.; Ni, J.; Zhou, L.; Peng, B.; Zhu, Y.; Cui, L. Elevated Fasting Blood Glucose Is Predictive of Poor Outcome in Non-Diabetic Stroke Patients: A Sub-Group Analysis of SMART. PLoS ONE 2016, 11, e0160674. [Google Scholar] [CrossRef]
- Clark, A.M.; Hartling, L.; Vandermeer, B.; McAlister, F.A. Meta-Analysis: Secondary Prevention Programs for Patients with Coronary Artery Disease. Ann. Intern. Med. 2005, 143, 659. [Google Scholar] [CrossRef]
- Gregg, E.; Jakicic, J. Association of the Magnitude of Weight Loss and Changes in Physical Fitness with Long-Term Cardiovascular Disease Outcomes in Overweight or Obese People with Type 2 Diabetes: A Post-Hoc Analysis of the Look AHEAD Randomised Clinical Trial. Lancet Diabetes Endocrinol. 2016, 4, 913–921. [Google Scholar] [CrossRef]
- Grey, M.; Boland, E.A.; Davidson, M.; Li, J.; Tamborlane, W.V. Coping Skills Training for Youth with Diabetes Mellitus Has Long-Lasting Effects on Metabolic Control and Quality of Life. J. Pediatr. 2000, 137, 107–113. [Google Scholar] [CrossRef]
- Fildes, A.; Charlton, J.; Rudisill, C.; Littlejohns, P.; Prevost, A.T.; Gulliford, M.C. Probability of an Obese Person Attaining Normal Body Weight: Cohort Study Using Electronic Health Records. Am. J. Public Health 2015, 105, e54–e59. [Google Scholar] [CrossRef]
- Kempf, K.; Röhling, M.; Niedermeier, K.; Gärtner, B.; Martin, S. Individualized Meal Replacement Therapy Improves Clinically Relevant Long-Term Glycemic Control in Poorly Controlled Type 2 Diabetes Patients. Nutrients 2018, 10, 1022. [Google Scholar] [CrossRef]
- Hallberg, S.J.; Dockter, N.E.; Kushner, J.A.; Athinarayanan, S.J. Improving the Scientific Rigour of Nutritional Recommendations for Adults with Type 2 Diabetes: A Comprehensive Review of the American Diabetes Association Guideline-Recommended Eating Patterns. Diabetes Obes. Metab. 2019, 21, 1769–1779. [Google Scholar] [CrossRef]
- Anderson, J.W.; Conley, S.B.; Nicholas, A.S. One Hundred–Pound Weight Losses with an Intensive Behavioral Program: Changes in Risk Factors in 118 Patients with Long-Term Follow-Up. Am. J. Clin. Nutr. 2007, 86, 301–307. [Google Scholar] [CrossRef]
- Feingold, K.R.; Grunfeld, C. Introduction to Lipids and Lipoproteins; MDText.Com Inc.: South Dartmouth, MA, USA, 2018. Available online: www.ncbi.nlm.nih.gov (accessed on 5 December 2022).
- Finkelstein, E.A.; Kruger, E. Meta- and Cost-Effectiveness Analysis of Commercial Weight Loss Strategies. Obesity 2014, 22, 1942–1951. [Google Scholar] [CrossRef]
- Khera, R.; Murad, M.H.; Chandar, A.K.; Dulai, P.S.; Wang, Z.; Prokop, L.J.; Loomba, R.; Camilleri, M.; Singh, S. Association of Pharmacological Treatments for Obesity with Weight Loss and Adverse Events: A Systematic Review and Meta-Analysis. JAMA 2016, 315, 2424–2434. [Google Scholar] [CrossRef]
- Bomberg, E.M.; Ryder, J.R.; Brundage, R.C.; Straka, R.J.; Fox, C.K.; Gross, A.C.; Oberle, M.M.; Bramante, C.T.; Sibley, S.D.; Kelly, A.S. Precision Medicine in Adult and Pediatric Obesity: A Clinical Perspective. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819863022. [Google Scholar] [CrossRef]
- Riaz, H.; Khan, M.S.; Siddiqi, T.J.; Usman, M.S.; Shah, N.; Goyal, A.; Khan, S.S.; Mookadam, F.; Krasuski, R.A.; Ahmed, H. Association between Obesity and Cardiovascular Outcomes. JAMA 2018, 1, e183788. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Mottershead, T.A.; Ronksley, P.E.; Sigal, R.J.; Campbell, T.S.; Hemmelgarn, B.R. Motivational Interviewing to Improve Weight Loss in Overweight And/or Obese Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Obes. Rev. 2011, 12, 709–723. [Google Scholar] [CrossRef]
- De Stefani, F.D.C.; Pietraroia, P.S.; Fernandes-Silva, M.M.; Faria-Neto, J.; Baena, C.P. Observational Evidence for Unintentional Weight Loss in All-Cause Mortality and Major Cardiovascular Events: A Systematic Review and Meta-Analysis. Sci. Rep. 2018, 8, 15447. [Google Scholar] [CrossRef]
- Chen, C.; Ye, Y.; Zhang, Y.; Pan, X.-F.; Pan, A. Weight Change across Adulthood in Relation to All Cause and Cause Specific Mortality: Prospective Cohort Study. BMJ 2019, 367, l5584. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ratner, R.E.; Han, J.; Kim, D.D.; Fineman, M.S.; Baron, A.D. Effects of Exenatide (Exendin-4) on Glycemic Control and Weight over 30 Weeks in Metformin-Treated Patients with Type 2 Diabetes. Diabetes Care 2005, 28, 1092–1100. [Google Scholar] [CrossRef]
- Durrer Schutz, D.; Busetto, L.; Dicker, D.; Farpour-Lambert, N.; Pryke, R.; Toplak, H.; Widmer, D.; Yumuk, V.; Schutz, Y. European Practical and Patient-Centred Guidelines for Adult Obesity Management in Primary Care. Obes. Facts 2019, 12, 40–66. [Google Scholar] [CrossRef]
- Koliaki, C.; Spinos, T.; Spinou, Μ.; Brinia, Μ.-E.; Mitsopoulou, D.; Katsilambros, N. Defining the Optimal Dietary Approach for Safe, Effective and Sustainable Weight Loss in Overweight and Obese Adults. Healthcare 2018, 6, 73. [Google Scholar] [CrossRef]
- Spanos, D.; Melville, C.A.; Hankey, C.R. Weight Management Interventions in Adults with Intellectual Disabilities and Obesity: A Systematic Review of the Evidence. Nutr. J. 2013, 12, 132. [Google Scholar] [CrossRef] [Green Version]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean Diet and Survival in a Greek Population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Caprio, M.; Infante, M.; Moriconi, E.; Armani, A.; Fabbri, A.; Mantovani, G.; Mariani, S.; Lubrano, C.; Poggiogalle, E.; Migliaccio, S.; et al. Very-Low-Calorie Ketogenic Diet (VLCKD) in the Management of Metabolic Diseases: Systematic Review and Consensus Statement from the Italian Society of Endocrinology (SIE). J. Endocrinol. Investig. 2019, 42, 1365–1386. [Google Scholar] [CrossRef]
- Landry, M.J.; Crimarco, A.; Gardner, C.D. Benefits of Low Carbohydrate Diets: A Settled Question or Still Controversial? Curr. Obes. Rep. 2021, 10, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Koh, G. Clinical Evidence and Mechanisms of High-Protein Diet-Induced Weight Loss. J. Obes. Metab. Syndr. 2020, 29, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Wahba, I.M.; Mak, R.H. Obesity and Obesity-Initiated Metabolic Syndrome: Mechanistic Links to Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2007, 2, 550–562. [Google Scholar] [CrossRef]
- Masood, W.; Uppaluri, K.R.; Annamaraju, P. Ketogenic Diet. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2021. [Google Scholar]
- Higgins, J.P.T.; Green, S. Cochrane Collaboration. In Cochrane Handbook for Systematic Reviews of Interventions; Wiley-Blackwell: Chisester, UK, 2011. [Google Scholar]
- Tricò, D.; Moriconi, D.; Berta, R.; Baldi, S.; Quinones-Galvan, A.; Guiducci, L.; Taddei, S.; Mari, A.; Nannipieri, M. Effectsof Low-Carbohydrate versus Mediterranean Dietson Weight Loss, Glucose Metabolism, Insulin Kineticsand β-Cell Functionin Morbidly Obese Individuals. Nutrients 2021, 13, 1345. [Google Scholar] [CrossRef]
- Guldbrand, H.; Dizdar, B.; Bunjaku, B.; Lindström, T.; Bachrach-Lindström, M.; Fredrikson, M.; Östgren, C.J.; Nystrom, F.H. In Type 2D iabetes, Randomisationto Adviceto Followa Low-Carbohydrate Diet Transiently Improves Glycaemic Control Compared with Adviceto Followa Low-Fat Diet Producinga Similar Weight Loss. Diabetologia 2012, 55, 2118–2127. [Google Scholar] [CrossRef]
- Ge, L.; Sadeghirad, B.; Ball, G.D.C.; da Costa, B.R.; Hitchcock, C.L.; Svendrovski, A.; Kiflen, R.; Quadri, K.; Kwon, H.Y.; Karamouzian, M.; et al. Comparison of Dietary Macronutrient Patterns of 14 Popular Named Dietary Programmes for Weight and Cardiovascular Risk Factor Reduction in Adults: Systematic Reviewand Network Meta-Analysis of Randomised Trials. BMJ 2020, 369, m696. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Wong, J.M.W.; Kendall, C.W.C.; Esfahani, A.; Ng, V.W.Y.; Leong, T.C.K.; Faulkner, D.A.; Vidgen, E.; Paul, G.; Mukherjea, R.; et al. Effect of a6-Month Vegan Low-Carbohydrate (“Eco-Atkins”) Dieton Cardiovascular Risk Factors and Body Weightin Hyperlipidaemic Adults: A Randomised ControlledTrial. BMJ Open 2014, 4, e003505. [Google Scholar] [CrossRef] [PubMed]
- Haufe, S.; Utz, W.; Engeli, S.; Kast, P.; Böhnke, J.; Pofahl, M.; Traber, J.; Haas, V.; Hermsdorf, M.; Mähler, A.; et al. Left Ventricular Mass and Function with Reduced-Fator Reduced-Carbohydrate Hypocaloric Dietsin Overweight and ObeseSubjects. Hypertension 2012, 59, 70–75. [Google Scholar] [CrossRef] [Green Version]
- Gardner, C.D.; Trepanowski, J.F.; DelGobbo, L.C.; Hauser, M.E.; Rigdon, J.; Ioannidis, J.P.A.; Desai, M.; King, A.C. Effect of Low-Fatvs Low-Carbohydrate Dieton 12-Month Weight Lossin Overweight Adults and the Association with Genotype Patternor Insulin Secretion. JAMA 2018, 319, 667. [Google Scholar] [CrossRef]
- Bazzano, L.A.; Hu, T.; Reynolds, K.; Yao, L.; Bunol, C.; Liu, Y.; Chen, C.-S.; Klag, M.J.; Whelton, P.K.; He, J. Effects of Low-Carbohydrate and Low-Fat Diets. Ann. Intern. Med. 2014, 161, 309. [Google Scholar] [CrossRef]
- deLuis, D.A.; Izaola, O.; Aller, R.; dela Fuente, B.; Bachiller, R.; Romero, E. Effectsofa High-Protein/Low Carbohydrateversusa Standard Hypocaloric Dieton Adipocytokine Levels and Insulin Resistance in Obese Patients along 9 months. J. Diabetes Complicat. 2015, 29, 950–954. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Hao, T.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Changes in Diet and Lifestyle and Long-Term Weight Gain in Women and Men. N. Engl. J. Med. 2011, 364, 2392–2404. [Google Scholar] [CrossRef]
- Foster, G.D. Weight and Metabolic Outcomes after 2 Years on a Low-Carbohydrate versus Low-Fat Diet. Ann. Intern. Med. 2010, 153, 147. [Google Scholar] [CrossRef]
- Cheng, A.Y.; Leiter, L.A. Implications of Recent Clinical Trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Curr. Opin. Intern. Med. 2006, 5, 461–465. [Google Scholar] [CrossRef]
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Weight Loss with a Low-Carbohydrate, Mediterranean, or Low-FatDiet. Obs. Gynecol. Surv. 2008, 63, 713–714. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Wong, J.M.W.; Kendall, C.W.C.; Esfahani, A.; Ng, V.W.Y.; Leong, T.C.K.; Faulkner, D.A.; Vidgen, E.; Greaves, K.A.; Paul, G.; et al. The Effect of a Plant-Based Low-Carbohydrate (“Eco-Atkins”) Diet on Body Weight and Blood Lipid Concentrations in Hyperlipidemic Subjects. Arch. Intern. Med. 2009, 169, 1046. [Google Scholar] [CrossRef]
- Bettini, S.; Belligoli, A.; Fabris, R.; Busetto, L. Diet Approach before and after Bariatric Surgery. Rev. Endocr. Metab Disord. 2020, 21, 297–306. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Kendall, C.W.C.; Marchie, A. Diet and Cholesterol Reduction. Ann. Intern. Med. 2005, 142, 793. [Google Scholar] [CrossRef] [PubMed]
- Corkey, B.E. Banting Lecture 2011: Hyperinsulinemia: Cause or Consequence? Diabetes 2011, 61, 4–13. [Google Scholar] [CrossRef]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition Therapy for Adults with Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef]
- McAuley, K.A.; Hopkins, C.M.; Smith, K.J.; McLay, R.T.; Williams, S.M.; Taylor, R.W.; Mann, J.I. Comparison of High-Fat and High-Protein Diets with a High-Carbohydrate Diet in Insulin-Resistant Obese Women. Diabetologia 2004, 48, 8–16. [Google Scholar] [CrossRef]
- Stentz, F.B.; Mikhael, A.; Kineish, O.; Christman, J.; Sands, C. High Protein Diet Leads to Prediabetes Remission and Positive Changes in Incretins and Cardiovascular Risk Factors. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1227–1237. [Google Scholar] [CrossRef]
- Kitabchi, A.E.; McDaniel, K.A.; Wan, J.Y.; Tylavsky, F.A.; Jacovino, C.A.; Sands, C.W.; Nyenwe, E.A.; Stentz, F.B. Effects of High-Protein versus High-Carbohydrate Diets on Markers of -Cell Function, Oxidative Stress, Lipid Peroxidation, Proinflammatory Cytokines, and Adipokines in Obese, Premenopausal Women without Diabetes: A Randomized Controlled Trial. Diabetes Care 2013, 36, 1919–1925. [Google Scholar] [CrossRef]
- Paoli, A.; Bianco, A.; Grimaldi, K.; Lodi, A.; Bosco, G. Long Term Successful Weight Loss with a Combination Biphasic Ketogenic Mediterranean Diet and Mediterranean Diet Maintenance Protocol. Nutrients 2013, 5, 5205–5217. [Google Scholar] [CrossRef]
- Ludwig, D.S. The Ketogenic Diet: Evidence for Optimism but High-Quality Research Needed. J. Nutr. 2019, 150, 1354–1359. [Google Scholar] [CrossRef]
- Barber, T.M.; Hanson, P.; Kabisch, S.; Pfeiffer, A.F.; Weickert, M.O. The Low-Carbohydrate Diet: Short-Term Metabolic Efficacy versus Longer-Term Limitations. Nutrients 2021, 13, 1187. [Google Scholar] [CrossRef]
- Moyers Scott, P. Which Diet Is Better—Low-Fat or Low-Carb? JAAPA 2006, 19, 49. [Google Scholar] [CrossRef]
- Schutz, Y.; Montani, J.P.; Dulloo, A.G. Low-Carbohydrate Ketogenic Diets in Body Weight Control: A Recurrent Plaguing Issue of Fad Diets? Obes. Rev. 2021, 22, e13195. [Google Scholar] [CrossRef]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary Carbohydrate Intake and Mortality: A Prospective Cohort Study and Meta-Analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef]
- Dreher, M. Whole Fruits and Fruit Fiber Emerging Health Effects. Nutrients 2018, 10, 1833. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Goldenberg, J.Z.; Day, A.; Brinkworth, G.D.; Sato, J.; Yamada, S.; Jönsson, T.; Beardsley, J.; Johnson, J.A.; Thabane, L.; Johnston, B.C. Efficacy and Safety of Low and Very Low Carbohydrate Diets for Type 2 Diabetes Remission: Systematic Review and Meta-Analysis of Published and Unpublished Randomized Trial Data. BMJ 2021, 372, m4743. [Google Scholar] [CrossRef] [PubMed]
- Thom, G.; Lean, M. Is There an Optimal Diet for Weight Management and Metabolic Health? Gastroenterology 2017, 152, 1739–1751. [Google Scholar] [CrossRef]
- Silverii, G.A.; Cosentino, C.; Santagiuliana, F.; Rotella, F.; Benvenuti, F.; Mannucci, E.; Cresci, B. Effectiveness of Low-Carbohydrate Diets for Long-Term Weight Loss in Obese Individuals: A Meta-Analysis of Randomized Controlled Trials. Diabetes Obes. Metab. 2022, 24, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Schugar, R.C.; Crawford, P.A. Low-Carbohydrate Ketogenic Diets, Glucose Homeostasis, and Nonalcoholic Fatty Liver Disease. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 374–380. [Google Scholar] [CrossRef]
- Stewart, W.A.; Gordon, K.; Camfield, P. Acute Pancreatitis Causing Death in a Child on the Ketogenic Diet. J. Child Neurol. 2001, 16, 682. [Google Scholar] [CrossRef]
- Bank, I.M.; Shemie, S.D.; Rosenblatt, B.; Bernard, C.; Mackie, A.S. Sudden Cardiac Death in Association with the Ketogenic Diet. Pediatr. Neurol. 2008, 39, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Norwitz, N.G.; Feldman, D.; Soto-Mota, A.; Kalayjian, T.; Ludwig, D.S. Elevated LDL Cholesterol with a Carbohydrate-Restricted Diet: Evidence for a “Lean Mass Hyper-Responder” Phenotype. Curr. Dev. Nutr. 2022, 6, 6001007. [Google Scholar] [CrossRef]
- Bostock, E.C.; Kirkby, K.C.; Taylor, B.V.; Hawrelak, J.A. Consumer Reports of “Keto Flu” Associated with the Ketogenic Diet. Front. Nutr. 2020, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Mancin, L.; Bianco, A.; Thomas, E.; Mota, J.F.; Piccini, F. Ketogenic Diet and Microbiota: Friends or Enemies? Genes 2019, 10, 534. [Google Scholar] [CrossRef] [PubMed]
- Raffensperger, J.F. The Least-Cost Low-Carbohydrate Diet Is Expensive. Nutr. Res. 2008, 28, 6–12. [Google Scholar] [CrossRef] [PubMed]

| Study Type | Measured Parameters | Number of Patients | Duration | Effects | References |
|---|---|---|---|---|---|
| Randomized clinical trial | Body weight, Insulin secretion, insulin clearance, β-cell function | 36 | 4 weeks | The mean body mass lowering was 5%, being 58% higher in the LCD group compared to Mediterranean-group. Fasting plasma glucose and glucose resistance remained unaltered by the nutritional interventions. The two nutritional interventions showed the same efficiency in improving insulin tolerance and fasting hyperinsulinemia while increasing endogenous insulin clearance and β-cell glucose sensitivity. | Tricò et al. [29] |
| A prospective randomized parallel trial | Body weight, HbA1c, HDL, LDL, Insulin doses | 61 | 2 years | At 2 years participants had an average weight loss of 5.1 kg. HbA1c fell in the LCD group only. At 6 months, HDL-cholesterol enhanced with the LCD, while LDL-cholesterol was not different between groups. Insulin doses were decreased in the LCD group. | Guldbrand et al. [30] |
| Randomized controlled trial | Body mass reduction, LDL-C, Triglyceride, Cholesterol, Apolipoprotein B:A1 ratios | 39 | 6 months | LCD showed a decrease LDL-C and triglyceride compared to low-carbohydrate treatment | Jenkins et al. [32] |
| Randomized controlled trial | Age, Body weight, BMI, overall body fat mass, waist circumference, blood lipids, triglycerides, cholesterol, HDL, LDL, Adiponectin, Leptin | 170 | 6 months | Subjects lost 7.3 ± 4.0 kg loss of weight with a reduced-carbohydrate diet and 6.2 ± 4.2 kg with a reduced-fat diet (p < 0.001) within each group. Calories restriction results in identical considerable reductions in left ventricular mass with high carbs diets (5.4 ± 5.4 g) or low-fat diets (5.2 ± 4.8 g; p < 0.001) within each group. | Haufe et al. [33] |
| Randomized clinical trial | Body weight, insulin secretion | 609 | 12 months | Weight alteration at 1st year, was −6.0 kg for the healthy LCD. There was no considerable nutrition–genotype pattern interplay or nutrition–insulin secretion interplay within the 1st year of body mass lowering | Gardner et al. [34] |
| Randomized parallel-group trial | Body weight, fat mass, HDL, cholesterol, triglycerides | 148 | 12 months | At 1st year, individuals on the LCD showed higher reductions in body mass, fat mass, a ratio of overall HDL cholesterol, and triglyceride concentrations and higher enhancement in HDL cholesterol concentrations compared to a low-fat diet. | Bazzano et al. [35] |
| Randomized clinical trial | BMI, body mass, fat mass, waist circumference, waist-to-hip ratio, systolic blood pressure, total cholesterol, LDL-cholesterol, insulin, Homeostatic Model Assessment for Insulin Resistance (HOMA) | 331 | 9 months | The reduction at 9 months of BMI, weight, fat mass, systolic blood pressure, insulin levels and HOMA were higher in the diet with low carbs than the diet with no carbs. With both interventions, leptin concentration was reduced. | de Luis et al. [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlidou, E.; Papadopoulou, S.K.; Fasoulas, A.; Mantzorou, M.; Giaginis, C. Clinical Evidence of Low-Carbohydrate Diets against Obesity and Diabetes Mellitus. Metabolites 2023, 13, 240. https://doi.org/10.3390/metabo13020240
Pavlidou E, Papadopoulou SK, Fasoulas A, Mantzorou M, Giaginis C. Clinical Evidence of Low-Carbohydrate Diets against Obesity and Diabetes Mellitus. Metabolites. 2023; 13(2):240. https://doi.org/10.3390/metabo13020240
Chicago/Turabian StylePavlidou, Eleni, Sousana K. Papadopoulou, Aristeidis Fasoulas, Maria Mantzorou, and Constantinos Giaginis. 2023. "Clinical Evidence of Low-Carbohydrate Diets against Obesity and Diabetes Mellitus" Metabolites 13, no. 2: 240. https://doi.org/10.3390/metabo13020240
APA StylePavlidou, E., Papadopoulou, S. K., Fasoulas, A., Mantzorou, M., & Giaginis, C. (2023). Clinical Evidence of Low-Carbohydrate Diets against Obesity and Diabetes Mellitus. Metabolites, 13(2), 240. https://doi.org/10.3390/metabo13020240