Serum Androgen Metabolites Correlate with Clinical Variables in African and European American Men with Localized, Therapy Naïve Prostate Cancer
Abstract
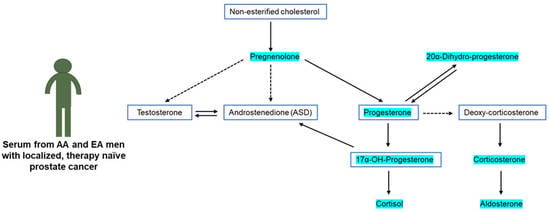
:1. Introduction
2. Materials and Methods
2.1. Liquid Chromatographic Assay with Tandem Mass Spectral Detection (LC-MS/MS)
2.2. Statistical Analyses
2.2.1. Patient Demographics and Comparisons of Clinical Variables between AA and EA Men
2.2.2. Univariate Comparisons of Serum Androgen Metabolite Levels and Clinical Cofactors
2.2.3. Multivariate Comparisons of Serum Androgen Metabolite Levels
2.2.4. Survival Analysis and Hazard Ratios
3. Results
3.1. Intermediate Metabolites Produced in the Primary Androgen Biosynthesis Pathway Are Associated with Age and Gleason Score in a Race-Independent Manner
3.2. Androgens and Intermediate Metabolite Levels in the Alternate Pathway Are Associated with Clinical Variables of Prostate Cancer
3.3. Serum Androgen Metabolites Correlate with Progression Free Survival (PFS)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fiandalo, M.V.; Wilton, J.; Mohler, J.L. Roles for the backdoor pathway of androgen metabolism in prostate cancer response to castration and drug treatment. Int. J. Biol. Sci. 2014, 10, 596–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, C.; Heemers, H.; Sharifi, N. Androgen Signaling in Prostate Cancer. Cold Spring Harb. Perspect. Med. 2017, 7, a030452. [Google Scholar] [CrossRef] [Green Version]
- Vander Griend, D.J.; Litvinov, I.V.; Isaacs, J.T. Conversion of androgen receptor signaling from a growth suppressor in normal prostate epithelial cells to an oncogene in prostate cancer cells involves a gain of function in c-Myc regulation. Int. J. Biol. Sci. 2014, 10, 627–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarif, J.C.; Lamb, L.E.; Schulz, V.V.; Nollet, E.A.; Miranti, C.K. Androgen receptor non-nuclear regulation of prostate cancer cell invasion mediated by Src and matriptase. Oncotarget 2015, 6, 6862–6876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinlein, C.A.; Chang, C. Androgen Receptor in Prostate Cancer. Endocr. Rev. 2004, 25, 276–308. [Google Scholar] [CrossRef] [Green Version]
- Lonergan, P.E.; Tindall, D.J. Androgen receptor signaling in prostate cancer development and progression. J. Carcinog. 2011, 10, 20. [Google Scholar] [CrossRef]
- Fiandalo, M.V.; Wu, W.; Mohler, J.L. The role of intracrine androgen metabolism, androgen receptor and apoptosis in the survival and recurrence of prostate cancer during androgen deprivation therapy. Curr. Drug Targets 2013, 14, 420–440. [Google Scholar] [CrossRef]
- Mostaghel, E.A.; Nelson, P.S. Intracrine androgen metabolism in prostate cancer progression: Mechanisms of castration resistance and therapeutic implications. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 243–258. [Google Scholar] [CrossRef] [Green Version]
- Hearn, J.W.D.; Sweeney, C.J.; Almassi, N.; Reichard, C.A.; Reddy, C.A.; Li, H.; Hobbs, B.; Jarrard, D.F.; Chen, Y.-H.; Dreicer, R.; et al. HSD3B1 Genotype and Clinical Outcomes in Metastatic Castration-Sensitive Prostate Cancer. JAMA Oncol. 2020, 6, e196496. [Google Scholar] [CrossRef]
- Sharifi, N. Minireview: Androgen Metabolism in Castration-Resistant Prostate Cancer. Mol. Endocrinol. 2013, 27, 708–714. [Google Scholar] [CrossRef] [Green Version]
- Litman, H.J.; Bhasin, S.; Link, C.L.; Araujo, A.B.; McKinlay, J.B. Serum androgen levels in black, Hispanic, and white men. J. Clin. Endocrinol. Metab. 2006, 91, 4326–4334. [Google Scholar] [CrossRef] [Green Version]
- Kubricht, W.S., 3rd; Williams, B.J.; Whatley, T.; Pinckard, P.; Eastham, J.A. Serum testosterone levels in African-American and white men undergoing prostate biopsy. Urology 1999, 54, 1035–1038. [Google Scholar] [CrossRef]
- Ross, R.; Bernstein, L.; Judd, H.; Hanisch, R.; Pike, M.; Henderson, B. Serum testosterone levels in healthy young black and white men. J. Natl. Cancer Inst. 1986, 76, 45–48. [Google Scholar]
- Allott, E.H.; Howard, L.E.; Aronson, W.J.; Terris, M.K.; Kane, C.J.; Amling, C.L.; Cooperberg, M.R.; Freedland, S.J. Racial Differences in the Association Between Preoperative Serum Cholesterol and Prostate Cancer Recurrence: Results from the SEARCH Database. Cancer Epidemiol. Biomark. Prev. 2016, 25, 547–554. [Google Scholar] [CrossRef] [Green Version]
- Jamnagerwalla, J.; Howard, L.E.; Allott, E.H.; Vidal, A.C.; Moreira, D.M.; Castro-Santamaria, R.; Andriole, G.L.; Freeman, M.R.; Freedland, S.J. Serum cholesterol and risk of high-grade prostate cancer: Results from the REDUCE study. Prostate Cancer Prostatic Dis. 2018, 21, 252–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marks, L.S.; Hess, D.L.; Dorey, F.J.; Macairan, M.L. Prostatic tissue testosterone and dihydrotestosterone in African-American and white men. Urology 2006, 68, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Mohler, J.L.; Gaston, K.E.; Moore, D.T.; Schell, M.J.; Cohen, B.L.; Weaver, C.; Petrusz, P. Racial differences in prostate androgen levels in men with clinically localized prostate cancer. J. Urol. 2004, 171, 2277–2280. [Google Scholar] [CrossRef] [PubMed]
- Rouleau, M.; Neveu, B.; Caron, P.; Morin, F.; Toren, P.; Lacombe, L.; Turcotte, V.; Lévesque, É.; Guillemette, C.; Pouliot, F. Extensive Alteration of Androgen Precursor Levels after Castration in Prostate Cancer Patients and Their Association with Active Androgen Level. J. Urol. 2022, 208, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, A.; Piyarathna, D.W.B.; Gohlke, J.; Putluri, V.; Soni, T.; Lloyd, S.; Castro, P.; Pennathur, S.; Jones, J.A.; Ittmann, M.; et al. Lipid Alterations in African American Men with Prostate Cancer. Metabolites 2021, 12, 8. [Google Scholar] [CrossRef]
- Berchuck, J.E.; Adib, E.; Abou Alaiwi, S.; Dash, A.K.; Shin, J.N.; Lowder, D.; McColl, C.; Castro, P.; Carelli, R.; Benedetti, E.; et al. The Prostate Cancer Androgen Receptor Cistrome in African American Men Associates with Upregulation of Lipid Metabolism and Immune Response. Cancer Res. 2022, 82, 2848–2859. [Google Scholar] [CrossRef]
- Koga, Y.; Song, H.; Chalmers, Z.R.; Newberg, J.; Kim, E.; Carrot-Zhang, J.; Piou, D.; Polak, P.; Abdulkadir, S.A.; Ziv, E.; et al. Genomic Profiling of Prostate Cancers from Men with African and European Ancestry. Clin. Cancer Res. 2020, 26, 4651–4660. [Google Scholar] [CrossRef] [PubMed]
- Jaratlerdsiri, W.; Chan, E.K.F.; Gong, T.; Petersen, D.C.; Kalsbeek, A.M.F.; Venter, P.A.; Stricker, P.D.; Bornman, M.S.R.; Hayes, V.M. Whole-Genome Sequencing Reveals Elevated Tumor Mutational Burden and Initiating Driver Mutations in African Men with Treatment-Naive, High-Risk Prostate Cancer. Cancer Res. 2018, 78, 6736–6746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayford, W.; Beksac, A.T.; Alger, J.; Alshalalfa, M.; Ahmed, M.; Khan, I.; Falagario, U.G.; Liu, Y.; Davicioni, E.; Spratt, D.E.; et al. Comparative analysis of 1152 African-American and European-American men with prostate cancer identifies distinct genomic and immunological differences. Commun. Biol. 2021, 4, 670. [Google Scholar] [CrossRef]
- Sanchez, T.W.; Zhang, G.; Li, J.; Dai, L.; Mirshahidi, S.; Wall, N.R.; Yates, C.; Wilson, C.; Montgomery, S.; Zhang, J.-Y.; et al. Immunoseroproteomic Profiling in African American Men with Prostate Cancer: Evidence for an Autoantibody Response to Glycolysis and Plasminogen-Associated Proteins. Mol. Cell. Proteom. 2016, 15, 3564–3580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooker, S.E., Jr.; Woods-Burnham, L.; Bathina, M.; Lloyd, S.; Gorjala, P.; Mitra, R.; Nonn, L.; Kimbro, K.S.; Kittles, R.A. Genetic Ancestry Analysis Reveals Misclassification of Commonly Used Cancer Cell Lines. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1003–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilton, J.H.; Titus, M.A.; Efstathiou, E.; Fetterly, G.J.; Mohler, J.L. Androgenic biomarker profiling in human matrices and cell culture samples using high throughput, electrospray tandem mass spectrometry. Prostate 2014, 74, 722–731. [Google Scholar] [CrossRef] [Green Version]
- Crawford, E.D.; Barqawi, A.B.; O’Donnell, C.; Morgentaler, A. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007, 100, 509–513. [Google Scholar] [CrossRef]
- Croghan, W.; Egeghy, P.P. Methods of Dealing with Values below the Limit of Detection Using SAS Carry. 2003. Available online: https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NERL&dirEntryId=64046 (accessed on 14 June 2021).
- Strushkevich, N.; Gilep, A.A.; Shen, L.; Arrowsmith, C.H.; Edwards, A.M.; Usanov, S.A.; Park, H.W. Structural insights into aldosterone synthase substrate specificity and targeted inhibition. Mol. Endocrinol. 2013, 27, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Zang, T.; Taplin, M.-E.; Tamae, D.; Xie, W.; Mesaros, C.; Zhang, Z.; Bubley, G.; Montgomery, B.; Balk, S.P.; Mostaghel, E.A.; et al. Testicular vs adrenal sources of hydroxy-androgens in prostate cancer. Endocr. Relat. Cancer 2017, 24, 393–404. [Google Scholar] [CrossRef] [Green Version]
- Hsing, A.W.; Stanczyk, F.Z.; Bélanger, A.; Schroeder, P.; Chang, L.; Falk, R.T.; Fears, T.R. Reproducibility of Serum Sex Steroid Assays in Men by RIA and Mass Spectrometry. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1004–1008. [Google Scholar] [CrossRef] [Green Version]
- Asbell, S.O.; Raimane, K.C.; Montesano, A.T.; Zeitzer, K.L.; Asbell, M.D.; Vijayakumar, S. Prostate-specific antigen and androgens in African-American and white normal subjects and prostate cancer patients. J. Natl. Med. Assoc. 2000, 92, 445–449. [Google Scholar] [PubMed]
- Osegbe, D.N.; Ogunlewe, J.O. Androgen concentration in blacks with benign and malignant prostatic disease. J. Urol. 1988, 140, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Quadri, S.F.; Dong, L.; Ignacio, L.; Kathuria, I.N.; Sutton, H.; Halpern, H. Results of a study to correlate serum prostate specific antigen and reproductive hormone levels in patients with localized prostate cancer. J. Natl. Med. Assoc. 1995, 87, 813–819. [Google Scholar] [PubMed]
- Ishizaki, F.; Nishiyama, T.; Kawasaki, T.; Miyashiro, Y.; Hara, N.; Takizawa, I.; Naito, M.; Takahashi, K. Androgen deprivation promotes intratumoral synthesis of dihydrotestosterone from androgen metabolites in prostate cancer. Sci. Rep. 2013, 3, 1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Liu, R.; Wang, L. Androgen metabolism genes in prostate cancer health disparities. Cancer Health Disparit. 2017, 1, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Winters, S.J.; Brufsky, A.; Weissfeld, J.; Trump, D.L.; Dyky, M.A.; Hadeed, V. Testosterone, sex hormone-binding globulin, and body composition in young adult African American and Caucasian men. Metabolism 2001, 50, 1242–1247. [Google Scholar] [CrossRef]
- Wu, A.H.; Whittemore, A.S.; Kolonel, L.N.; John, E.M.; Gallagher, R.P.; West, D.W.; Hankin, J.; Teh, C.Z.; Dreon, D.M.; Paffenbarger, R.S., Jr. Serum androgens and sex hormone-binding globulins in relation to lifestyle factors in older African-American, white, and Asian men in the United States and Canada. Cancer Epidemiol. Biomark. Prev. 1995, 4, 735–741. [Google Scholar]
- Lopez, D.S.; Peskoe, S.B.; Joshu, C.E.; Dobs, A.; Feinleib, M.; Kanarek, N.; Nelson, W.G.; Selvin, E.; Rohrmann, S.; Platz, E.A. Racial/ethnic differences in serum sex steroid hormone concentrations in US adolescent males. Cancer Causes Control 2013, 24, 817–826. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Chen, X.; Hu, H.; Dailey, A.B.; Taylor, B.D. Current opinion on the role of testosterone in the development of prostate cancer: A dynamic model. BMC Cancer 2015, 15, 806. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Chen, X.; Bird, V.Y.; Gerke, T.A.; Manini, T.M.; Prosperi, M. Association between age-related reductions in testosterone and risk of prostate cancer-An analysis of patients’ data with prostatic diseases. Int. J. Cancer 2017, 141, 1783–1793. [Google Scholar] [CrossRef]
- Le Floch, E.; Cosentino, T.; Larsen, C.K.; Beuschlein, F.; Reincke, M.; Amar, L.; Rossi, G.-P.; De Sousa, K.; Baron, S.; Chantalat, S.; et al. Identification of risk loci for primary aldosteronism in genome-wide association studies. Nat. Commun. 2022, 13, 5198. [Google Scholar] [CrossRef] [PubMed]
- Mahal, B.A.; Berman, R.A.; Taplin, M.; Huang, F.W. Prostate cancer–specific mortality across gleason scores in black vs nonblack men. JAMA 2018, 320, 2479–2481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeSantis, C.E.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Cancer statistics for African Americans, 2019. CA Cancer J. Clin. 2019, 69, 211–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Respond, African American Prostate Cancer Study. Available online: https://respondstudy.org/ (accessed on 15 September 2021).
- Prostate Cancer Foundation, Smith Polygenic Risk Test for Prostate Cancer. Available online: https://www.pcf.org/sprt/ (accessed on 15 September 2021).
- Awasthi, S.; Berglund, A.; Abraham-Miranda, J.; Rounbehler, R.J.; Kensler, K.; Serna, A.; Vidal, A.; You, S.; Freeman, M.R.; Davicioni, E.; et al. Comparative Genomics Reveals Distinct Immune-oncologic Pathways in African American Men with Prostate Cancer. Clin. Cancer Res. 2021, 27, 320–329. [Google Scholar] [CrossRef]
- Yuan, J.; Kensler, K.H.; Hu, Z.; Zhang, Y.; Zhang, T.; Jiang, J.; Xu, M.; Pan, Y.; Long, M.; Montone, K.T.; et al. Integrative comparison of the genomic and transcriptomic landscape between prostate cancer patients of predominantly African or European genetic ancestry. PLoS Genet. 2020, 16, e1008641. [Google Scholar] [CrossRef] [Green Version]




| Metabolite (Adjusted for Age and Grade) | EA Mean (Std Error) | AA Mean (Std Error) | p-Value |
|---|---|---|---|
| 17α-OH-Progesterone (ng/mL) | 0.83 (0.05) | 0.68 (0.06) | 0.043 |
| 20α-Dihyro-Progesterone (ng/mL) | 0.06 (0.00) | 0.05 (0.00) | 0.071 |
| Aldosterone (ng/mL) | 0.13 (0.02) | 0.23 (0.03) | 0.005 |
| All observed Androsterone (AND) (ng/mL) | 0.13 (0.01) | 0.12 (0.01) | 0.543 |
| Androstenedione (ASD) (ng/mL) | 0.64 (0.05) | 0.55 (0.05) | 0.206 |
| Corticosterone (ng/mL) | 3.58 (0.37) | 1.86 (0.42) | 0.001 |
| Cortisol (ng/mL) | 83.18 (3.59) | 66.18 (4.03) | 0.001 |
| Cortisone (ng/mL) | 15.65 (0.63) | 14.60 (0.71) | 0.254 |
| Dehydroepiandrosterone (DHEA) (ng/mL) | 1.62 (0.16) | 1.24 (0.18) | 0.094 |
| Dihydrotestosterone (DHT) (ng/mL) | 0.38 (0.03) | 0.38 (0.04) | 0.969 |
| Deoxy-Corticosterone (ng/mL) | 0.04 (0.00) | 0.03 (0.00) | 0.032 |
| Dihydro-Progesterone (ng/mL) | 0.03 (0.00) | 0.03 (0.00) | 0.742 |
| Epi-Androsterone (ng/mL) | 0.18 (0.02) | 0.19 (0.02) | 0.836 |
| Epi-Testosterone (ng/mL) | 0.04 (0.00) | 0.05 (0.00) | 0.763 |
| Estrone (ng/mL) | 0.03 (0.00) | 0.04 (0.00) | 0.050 |
| Hydroxy Pregnenolone (ng/mL) | 1.48 (0.16) | 1.04 (0.18) | 0.052 |
| Non-esterified Cholesterol (µg/mL) | 97.29 (8.61) | 85.21 (9.67) | 0.332 |
| Pregnenolone (ng/mL) | 0.70 (0.04) | 0.59 (0.04) | 0.044 |
| Progesterone (ng/mL) | 0.04 (0.00) | 0.03 (0.00) | 0.105 |
| Sex Hormone Binding Globulin (SHBG) (nmol/L) | 41.12 (3.03) | 42.99 (3.41) | 0.670 |
| Testosterone (T) (ng/mL) | 4.28 (0.25) | 3.73 (0.28) | 0.135 |
| Metabolite (adjusted for PSA and grade) | <=3 + 4 | >=4 + 3 | p-value |
| 17α-OH-Progesterone (ng/mL) | 0.82 (0.03) | 0.63 (0.07) | 0.044 |
| 20α-Dihyro-Progesterone (ng/mL) | 0.06 (0.00) | 0.05 (0.01) | 0.025 |
| Aldosterone (ng/mL) | 0.17 (0.06) | 0.17 (0.13) | 1.000 |
| All observed Androsterone (AND) (ng/mL) | 0.13 (0.01) | 0.07 (0.01) | 0.001 |
| Androstenedione (ASD) (ng/mL) | 0.66 (0.03) | 0.10 (0.06) | <0.001 |
| Corticosterone (ng/mL) | 2.82 (0.32) | 1.42 (0.65) | 0.101 |
| Cortisol (ng/mL) | 89.07 (2.69) | 41.09 (5.39) | <0.001 |
| Cortisone (ng/mL) | 17.64 (0.53) | 8.06 (1.07) | <0.001 |
| Dehydroepiandrosterone (DHEA) (ng/mL) | 1.59 (0.14) | BLQ (0.28) | <0.001 |
| Dihydrotestosterone (DHT) (ng/mL) | 0.39 (0.02) | 0.50 (0.04) | 0.045 |
| Deoxy-Corticosterone (ng/mL) | 0.04 (0.00) | 0.02 (0.01) | 0.012 |
| Dihydro-Progesterone (ng/mL) | 0.03 (0.00) | 0.03 (0.00) | 0.810 |
| Epi-Androsterone (ng/mL) | 0.12 (0.01) | 0.19 (0.02) | 0.008 |
| Epi-Testosterone (ng/mL) | 0.04 (0.00) | 0.04 (0.00) | 0.885 |
| Estrone (ng/mL) | 0.04 (0.00) | 0.03 (0.00) | 0.284 |
| Hydroxy Pregnenolone (ng/mL) | 1.22 (0.14) | BLQ (0.29) | <0.001 |
| Non-esterified Cholesterol (µg/mL) | 98.17 (1.44) | 96.24 (2.89) | 0.614 |
| Pregnenolone (ng/mL) | 0.65 (0.03) | 0.37 (0.06) | <0.001 |
| Progesterone (ng/mL) | 0.04 (0.00) | 0.03 (0.01) | 0.020 |
| Sex Hormone Binding Globulin (SHBG) (nmol/L) | 39.01 (1.65) | 55.27 (3.31) | <0.001 |
| Testosterone (T) (ng/mL) | 3.97 (0.16) | 4.25 (0.31) | 0.494 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramakrishnan, S.; Kittles, R.A.; Huss, W.J.; Wang, J.; Attwood, K.; Woloszynska, A. Serum Androgen Metabolites Correlate with Clinical Variables in African and European American Men with Localized, Therapy Naïve Prostate Cancer. Metabolites 2023, 13, 284. https://doi.org/10.3390/metabo13020284
Ramakrishnan S, Kittles RA, Huss WJ, Wang J, Attwood K, Woloszynska A. Serum Androgen Metabolites Correlate with Clinical Variables in African and European American Men with Localized, Therapy Naïve Prostate Cancer. Metabolites. 2023; 13(2):284. https://doi.org/10.3390/metabo13020284
Chicago/Turabian StyleRamakrishnan, Swathi, Rick A. Kittles, Wendy J. Huss, Jianmin Wang, Kristopher Attwood, and Anna Woloszynska. 2023. "Serum Androgen Metabolites Correlate with Clinical Variables in African and European American Men with Localized, Therapy Naïve Prostate Cancer" Metabolites 13, no. 2: 284. https://doi.org/10.3390/metabo13020284
APA StyleRamakrishnan, S., Kittles, R. A., Huss, W. J., Wang, J., Attwood, K., & Woloszynska, A. (2023). Serum Androgen Metabolites Correlate with Clinical Variables in African and European American Men with Localized, Therapy Naïve Prostate Cancer. Metabolites, 13(2), 284. https://doi.org/10.3390/metabo13020284