Association of Red Blood Cell Distribution Width and Neutrophil-to-Lymphocyte Ratio with Calcification and Cardiovascular Markers in Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Laboratory Analyses
2.3. PWV, Peripheral and Central Hemodynamic Parameters Measurement
2.4. Statistics
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parfrey, P.S.; Foley, R.N. The clinical epidemiology of cardiac disease in chronic renal failure. J. Am. Soc. Nephrol. 1999, 10, 1606–1615. [Google Scholar] [CrossRef]
- Foley, R.N.; Murray, A.M.; Li, S.; Herzog, C.A.; McBean, A.M.; Eggers, P.W.; Collins, A.J. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J. Am. Soc. Nephrol. 2005, 16, 489–495. [Google Scholar] [CrossRef]
- Russo, D.; Palmiero, G.; De Blasio, A.P.; Balletta, M.M.; Andreucci, V.E. Coronary artery calcification in patients with CRF not undergoing dialysis. Am. J. Kidney Dis. 2004, 44, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, S.; Roumeliotis, A.; Stamou, A.; Leivaditis, K.; Kantartzi, K.; Panagoutsos, S.; Liakopoulos, V. The association of dp-ucMGP with cardiovascular morbidity and decreased renal function in diabetic chronic kidney disease. Int. J. Mol. Sci. 2020, 21, 6035. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, S.; Roumeliotis, A.; Dounousi, E.; Eleftheriadis, T.; Liakopoulos, V. Biomarkers of vascular calcification in serum. Adv. Clin. Chem. 2020, 98, 91–147. [Google Scholar] [PubMed]
- Roumeliotis, S.; Dounousi, E.; Eleftheriadis, T.; Liakopoulos, V. Association of the inactive circulating matrix Gla protein with vitamin K intake, calcification, mortality, and cardiovascular disease: A review. Int. J. Mol. Sci. 2019, 20, 628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schurgers, L.J.; Barreto, D.V.; Barreto, F.C.; Liabeuf, S.; Renard, C.; Magdeleyns, E.J.; Vermeer, C.; Choukroun, G.; Massy, Z.A. The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: A preliminary report. Clin. J. Am. Soc. Nephrol. 2010, 5, 568–575. [Google Scholar] [CrossRef] [Green Version]
- Townsend, R.; Anderson, A.; Chirinos, J.; Feldman, H.; Grunwald, J.; Nessel, L.; Roy, J.; Weir, M.; Wright Jr, J.; Bansal, N. CRIC Study Investigators: Association of pulse wave velocity with chronic kidney disease progression and mortality: Findings from the CRIC study (Chronic Renal Insufficiency Cohort). Hypertension 2018, 71, 1101–1107. [Google Scholar] [CrossRef]
- Buttarello, M. Laboratory diagnosis of anemia: Are the old and new red cell parameters useful in classification and treatment, how? Int. J. Lab. Hematol. 2016, 38, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Zalawadiya, S.K.; Veeranna, V.; Niraj, A.; Pradhan, J.; Afonso, L. Red cell distribution width and risk of coronary heart disease events. Am. J. Cardiol. 2010, 106, 988–993. [Google Scholar] [CrossRef]
- van Kimmenade, R.R.; Mohammed, A.A.; Uthamalingam, S.; van der Meer, P.; Felker, G.M.; Januzzi, J.L., Jr. Red blood cell distribution width and 1-year mortality in acute heart failure. Eur. J. Heart Fail. 2010, 12, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Lappegård, J.; Ellingsen, T.S.; Hindberg, K.; Mathiesen, E.B.; Njølstad, I.; Wilsgaard, T.; Løchen, M.-L.; Brækkan, S.K.; Hansen, J.-B. Impact of chronic inflammation, assessed by hs-CRP, on the association between red cell distribution width and arterial cardiovascular disease: The Tromsø Study. TH Open 2018, 2, e182–e189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sotiropoulos, K.; Yerly, P.; Monney, P.; Garnier, A.; Regamey, J.; Hugli, O.; Martin, D.; Metrich, M.; Antonietti, J.P.; Hullin, R. Red cell distribution width and mortality in acute heart failure patients with preserved and reduced ejection fraction. ESC Heart Fail. 2016, 3, 198–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felker, G.M.; Allen, L.A.; Pocock, S.J.; Shaw, L.K.; McMurray, J.J.; Pfeffer, M.A.; Swedberg, K.; Wang, D.; Yusuf, S.; Michelson, E.L. Red cell distribution width as a novel prognostic marker in heart failure: Data from the CHARM Program and the Duke Databank. J. Am. Coll. Cardiol. 2007, 50, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Gao, B.; Wang, F.; Zhao, M.-h.; Wang, J.; Zhang, L. Red blood cell distribution width is associated with adverse kidney outcomes in patients with chronic kidney disease. Front. Med. 2022, 9. [Google Scholar] [CrossRef]
- Hsieh, Y.-P.; Chang, C.-C.; Kor, C.-T.; Yang, Y.; Wen, Y.-K.; Chiu, P.-F. The predictive role of red cell distribution width in mortality among chronic kidney disease patients. PLoS ONE 2016, 11, e0162025. [Google Scholar] [CrossRef] [Green Version]
- Roumeliotis, S.; Stamou, A.; Roumeliotis, A.; Theodoridis, M.; Leivaditis, K.; Panagoutsos, S.; Liakopoulos, V. Red blood cell distribution width is associated with deterioration of renal function and cardiovascular morbidity and mortality in patients with diabetic kidney disease. Life 2020, 10, 301. [Google Scholar] [CrossRef]
- Kor, C.-T.; Hsieh, Y.-P.; Chang, C.-C.; Chiu, P.-F. The prognostic value of interaction between mean corpuscular volume and red cell distribution width in mortality in chronic kidney disease. Sci. Rep. 2018, 8, 11870. [Google Scholar] [CrossRef] [Green Version]
- Solak, Y.; Gaipov, A.; Turk, S.; Kayrak, M.; Yilmaz, M.I.; Caglar, K.; Verim, S.; Unal, H.U.; Gok, M.; Demirkaya, E. Red cell distribution width is independently related to endothelial dysfunction in patients with chronic kidney disease. Am. J. Med. Sci. 2014, 347, 118–124. [Google Scholar] [CrossRef]
- Forget, P.; Khalifa, C.; Defour, J.-P.; Latinne, D.; Van Pel, M.-C.; De Kock, M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res. Notes 2017, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.-M.; Tao, S.-M.; Liu, G.-L. Neutrophil-to-lymphocyte ratio in relation to the risk of all-cause mortality and cardiovascular events in patients with chronic kidney disease: A systematic review and meta-analysis. Ren. Fail. 2020, 42, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J.; Balk, E.; Kausz, A.T.; Levin, A.; Steffes, M.W.; Hogg, R.J.; Perrone, R.D.; Lau, J.; Eknoyan, G. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann. Intern. Med. 2003, 139, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Workgroup, K. KDIGO clinical practice guideline for acute kidney injury. Nephron Clin. Pract. 2012, 2, 179–184. [Google Scholar]
- Jaminon, A.M.; Dai, L.; Qureshi, A.R.; Evenepoel, P.; Ripsweden, J.; Söderberg, M.; Witasp, A.; Olauson, H.; Schurgers, L.J.; Stenvinkel, P. Matrix Gla protein is an independent predictor of both intimal and medial vascular calcification in chronic kidney disease. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Vaios, V.; Georgianos, P.I.; Vareta, G.; Dounousi, E.; Eleftheriadis, T.; Papagianni, A.; Zebekakis, P.E.; Liakopoulos, V. A comparative analysis of ambulatory BP profile and arterial stiffness between CAPD and APD. Hum. Hypertens. 2022, 36, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Franssen, P.M.; Imholz, B.P. Evaluation of the Mobil-O-Graph new generation ABPM device using the ESH criteria. Blood Press. Monit. 2010, 15, 229–231. [Google Scholar] [CrossRef]
- Perlstein, T.S.; Weuve, J.; Pfeffer, M.A.; Beckman, J.A. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch. Intern. Med. 2009, 169, 588–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, L.A.; Felker, G.M.; Mehra, M.R.; Chiong, J.R.; Dunlap, S.H.; Ghali, J.K.; Lenihan, D.J.; Oren, R.M.; Wagoner, L.E.; Schwartz, T.A. Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J. Card. Fail. 2010, 16, 230–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Najjar, Y.; Goode, K.M.; Zhang, J.; Cleland, J.G.; Clark, A.L. Red cell distribution width: An inexpensive and powerful prognostic marker in heart failure. Eur. J. Heart Fail. 2009, 11, 1155–1162. [Google Scholar] [CrossRef]
- Ye, Z.; Smith, C.; Kullo, I.J. Usefulness of red cell distribution width to predict mortality in patients with peripheral artery disease. Am. J. Cardiol. 2011, 107, 1241–1245. [Google Scholar] [CrossRef] [Green Version]
- Azab, B.; Torbey, E.; Hatoum, H.; Singh, J.; Khoueiry, G.; Bachir, R.; McGinn, J.T., Jr.; McCord, D.; Lafferty, J. Usefulness of red cell distribution width in predicting all-cause long-term mortality after non-ST-elevation myocardial infarction. Cardiology 2011, 119, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Dabbah, S.; Hammerman, H.; Markiewicz, W.; Aronson, D. Relation between red cell distribution width and clinical outcomes after acute myocardial infarction. Am. J. Cardiol. 2010, 105, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Sun, T.; Li, C.; Li, Y.; Guo, Z.; Wang, W.; Li, D. An overall and dose-response meta-analysis of red blood cell distribution width and CVD outcomes. Sci. Rep. 2017, 7, 43420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukasawa, H.; Ishibuchi, K.; Kaneko, M.; Niwa, H.; Yasuda, H.; Kumagai, H.; Furuya, R. Red blood cell distribution width is associated with all-cause and cardiovascular mortality in hemodialysis patients. Ther. Apher. Dial. 2017, 21, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Vashistha, T.; Streja, E.; Molnar, M.Z.; Rhee, C.M.; Moradi, H.; Soohoo, M.; Kovesdy, C.P.; Kalantar-Zadeh, K. Red cell distribution width and mortality in hemodialysis patients. Am. J. Kidney Dis. 2016, 68, 110–121. [Google Scholar] [CrossRef] [Green Version]
- Sun, I.O.; Chung, B.H.; Yoon, H.J.; Kim, J.H.; Choi, B.S.; Park, C.W.; Kim, Y.S.; Yang, C.W.; Lee, K.Y. Clinical significance of red blood cell distribution width in the prediction of mortality in patients on peritoneal dialysis. Kidney Res. Clin. Pract. 2016, 35, 114–118. [Google Scholar] [CrossRef] [Green Version]
- Soohoo, M.; Molnar, M.Z.; Ujszaszi, A.; Obi, Y.; Kovesdy, C.P.; Kalantar-Zadeh, K.; Streja, E. Red blood cell distribution width and mortality and hospitalizations in peritoneal dialysis patients. Ephrol. Dial. Transplant. 2019, 34, 2111–2118. [Google Scholar] [CrossRef]
- Cao, H.-X.; Zhao, X.-d.; Yan, L.; Fan, X.-G.; Shao, F.-M. Correlation between red blood cell distribution width and cardiovascular events in the patients receiving peritoneal dialysis: A Strobe-compliant article. Medicine 2019, 98, e14376. [Google Scholar] [CrossRef]
- Zhang, T.; Li, J.; Lin, Y.; Yang, H.; Cao, S. Association between red blood cell distribution width and all-cause mortality in chronic kidney disease patients: A systematic review and meta-analysis. Arch. Med. Res. 2017, 48, 378–385. [Google Scholar] [CrossRef]
- den Harder, A.M.; de Jong, P.A.; de Groot, M.C.; Wolterink, J.M.; Budde, R.P.; Iŝgum, I.; van Solinge, W.W.; Maarten, J.; Lutgens, E.; Veldhuis, W.B. Commonly available hematological biomarkers are associated with the extent of coronary calcifications. Atherosclerosis 2018, 275, 166–173. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Dounousi, E.; Salmas, M.; Eleftheriadis, T.; Liakopoulos, V. Vascular calcification in chronic kidney disease: The role of vitamin K-dependent matrix Gla protein. Front. Med. 2020, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, S.; Duni, A.; Vaios, V.; Kitsos, A.; Liakopoulos, V.; Dounousi, E. Vitamin K Supplementation for Prevention of Vascular Calcification in Chronic Kidney Disease Patients: Are We There Yet? Nutrients 2022, 14, 925. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, S.; Roumeliotis, A.; Panagoutsos, S.; Giannakopoulou, E.; Papanas, N.; Manolopoulos, V.G.; Passadakis, P.; Tavridou, A. Matrix Gla protein T-138C polymorphism is associated with carotid intima media thickness and predicts mortality in patients with diabetic nephropathy. J. Diabetes Its Complicat. 2017, 31, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, S.; Roumeliotis, A.; Dounousi, E.; Eleftheriadis, T.; Liakopoulos, V. Vitamin K for the treatment of cardiovascular disease in End-Stage Renal Disease patients: Is there hope? Curr. Vasc. Pharmacol. 2021, 19, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, S.; Roumeliotis, A.; Georgianos, P.I.; Thodis, E.; Schurgers, L.J.; Maresz, K.; Eleftheriadis, T.; Dounousi, E.; Tripepi, G.; Mallamaci, F. VItamin K In PEritonial DIAlysis (VIKIPEDIA): Rationale and study protocol for a randomized controlled trial. PLoS ONE 2022, 17, e0273102. [Google Scholar] [CrossRef]
- Lappegård, J.; Ellingsen, T.S.; Vik, A.; Skjelbakken, T.; Brox, J.; Mathiesen, E.B.; Johnsen, S.H.; Brækkan, S.K.; Hansen, J.-B. Red cell distribution width and carotid atherosclerosis progression The Tromsø Study. Thromb. Haemost. 2015, 113, 649–654. [Google Scholar]
- Söderholm, M.; Borné, Y.; Hedblad, B.; Persson, M.; Engström, G. Red cell distribution width in relation to incidence of stroke and carotid atherosclerosis: A population-based cohort study. PLoS ONE 2015, 10, e0124957. [Google Scholar] [CrossRef] [Green Version]
- Sicaja, M.; Pehar, M.; Đerek, L.; Starčević, B.; Vuletić, V.; Romić, Ž.; Božikov, V. Red blood cell distribution width as a prognostic marker of mortality in patients on chronic dialysis: A single center, prospective longitudinal study. Croat. Med. J. 2013, 54, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Khalil, A.; Shehata, M.; Abdeltawab, A.; Onsy, A. Red blood cell distribution width and coronary artery disease severity in diabetic patients. Future Cardiol. 2019, 15, 355–366. [Google Scholar]
- Czyzewski, L.; Wyzgal, J.; Czyzewska, E.; Kurowski, A.; Sierdzinski, J.; Truszewski, Z.; Szarpak, L. Assessment of arterial stiffness, volume, and nutritional status in stable renal transplant recipients. Medicine 2016, 95, e2819. [Google Scholar] [CrossRef]
- Pacek, A.; Saran, M.; Wyzgał, J. 24-hour Arterial stiffness monitoring in kidney transplant recipients in the early postoperative period. Trans. Proc. 2018, 50, 1824–1828. [Google Scholar] [CrossRef] [PubMed]
- Fornal, M.; Wizner, B.; Cwynar, M.; Królczyk, J.; Kwater, A.; Korbut, R.A.; Grodzicki, T. Association of red blood cell distribution width, inflammation markers and morphological as well as rheological erythrocyte parameters with target organ damage in hypertension. Clin. Hemorheol. Microcirc. 2014, 56, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Del Giorno, R.; Troiani, C.; Gabutti, S.; Stefanelli, K.; Gabutti, L. Comparing oscillometric and tonometric methods to assess pulse wave velocity: A population-based study. Ann. Med. 2021, 53, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Giachelli, C.M.; Speer, M.Y.; Li, X.; Rajachar, R.M.; Yang, H. Regulation of vascular calcification: Roles of phosphate and osteopontin. Circ. Res. 2005, 96, 717–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giachelli, C.M. The emerging role of phosphate in vascular calcification. Kidney Int. 2009, 75, 890–897. [Google Scholar] [CrossRef] [Green Version]
- Shroff, R.; Long, D.A.; Shanahan, C. Mechanistic insights into vascular calcification in CKD. Am. Soc. Nephrol. 2013, 24, 179–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assulyn, T.; Khamisy-Farah, R.; Nseir, W.; Bashkin, A.; Farah, R. Neutrophil-to-lymphocyte ratio and red blood cell distribution width as predictors of microalbuminuria in type 2 diabetes. J. Clin. Lab. Anal. 2020, 34, e23259. [Google Scholar] [CrossRef] [Green Version]
- Uslu, A.U.; Yonem, O.; Aydin, B.; Uncu, T.; Seven, D.; Balta, S.; Cicekli, E. Red cell distribution width is associated with albuminuria in adults with familial Mediterranean fever. Kaohsiung J. Med. Sci. 2016, 32, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Peng, F.; Li, Z.; Zhong, Z.; Luo, Q.; Guo, Q.; Huang, F.; Yu, X.; Yang, X. An increasing of red blood cell distribution width was associated with cardiovascular mortality in patients on peritoneal dialysis. Int. J. Cardiol. 2014, 176, 1379–1381. [Google Scholar] [CrossRef]
- Li, W.-J.; Chen, X.-M.; Nie, X.-Y.; Zhang, J.; Cheng, Y.-J.; Lin, X.-X.; Wu, S.-H. Cardiac troponin and C-reactive protein for predicting all-cause and cardiovascular mortality in patients with chronic kidney disease: A meta-analysis. Clinics 2015, 70, 301–311. [Google Scholar] [CrossRef]
- Goicoechea, M.; Quiroga, B.; García de Vinuesa, S.; Verdalles, Ú.; Reque, J.; Panizo, N.; Arroyo, D.; Santos, A.; Macías, N.; Luño, J. Intraindividual interleukin-6 variations on the cardiovascular prognosis of patients with chronic renal disease. Ren. Fail. 2012, 34, 1002–1009. [Google Scholar] [CrossRef]
- Ao, G.; Wang, Y.; Qi, X.; Wang, F.; Wen, H. Association of neutrophil-to-lymphocyte ratio and risk of cardiovascular or all-cause mortality in chronic kidney disease: A meta-analysis. Clin. Exp. Nephrol. 2021, 25, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-r.; Yuan, L.-.; Shi, R.; Li, H.; Wang, D.-G.; Wu, Y.-G. Predictors of coronary artery calcification and its association with cardiovascular events in patients with chronic kidney disease. Ren. Fail. 2021, 43, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Hu, X.; Pan, H.; Xiao, Y.; Dong, J.; Bao, Y.; Fang, M. Cardiac valve calcification prevalence and association with neutrophil-to-lymphocyte ratio in newly diagnosed patients with non-dialysis chronic kidney disease stage 3-5. Bratisl. Med. J. 2022, 123, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, K.; Guney, I.; Yerlikaya, F.H.; Tonbul, H.Z. The relationship between neutrophil-to-lymphocyte ratio and inflammation in end-stage renal disease patients. Ren. Fail. 2012, 34, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Yaprak, M.; Turan, M.N.; Dayanan, R.; Akın, S.; Değirmen, E.; Yıldırım, M.; Turgut, F. Platelet-to-lymphocyte ratio predicts mortality better than neutrophil-to-lymphocyte ratio in hemodialysis patients. Int. Urol. Nephrol. 2016, 48, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Raj, G.; Awasthi, N.P.; Rao, N.; Srivastava, D. Evaluation of the relationship between blood cell parameters and vascular calcification in dialysis-dependent end-stage renal disease patients. Saudi J. Kidney Dis. Transplant. 2020, 31, 136. [Google Scholar]
- Shanahan, C.M.; Crouthamel, M.H.; Kapustin, A.; Giachelli, C.M. Arterial calcification in chronic kidney disease: Key roles for calcium and phosphate. Circ. Res. 2011, 109, 697–711. [Google Scholar] [CrossRef] [Green Version]
- Cai, K.; Luo, Q.; Zhu, B.; Han, L.; Wu, D.; Dai, Z.; Wang, K. Neutrophil-lymphocyte ratio is associated with arterial stiffness in patients with peritoneal dialysis. BMC Nephrol. 2016, 17, 191. [Google Scholar] [CrossRef] [Green Version]
- Delcea, C.; Buzea, C.A.; Dan, G.A. The neutrophil to lymphocyte ratio in heart failure: A comprehensive review. Rom. J. Intern. Med. 2019, 57, 296–314. [Google Scholar] [CrossRef] [Green Version]
- Durmus, E.; Kivrak, T.; Gerin, F.; Sunbul, M.; Sari, I.; Erdogan, O. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio are predictors of heart failure. Arq. Bras. Cardiol. 2015, 105, 606–613. [Google Scholar] [CrossRef]
- Li, Q.; Shi, S.; Liu, L.; Lv, J.; Zhu, L.; Zhang, H. Neutrophil-to-lymphocyte ratio as an independent inflammatory indicator for poor renal prognosis in adult IgA vasculitis with nephritis. Int. Immunopharmacol. 2022, 111, 109178. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Tang, Q.; Zhang, L.; Wang, H. High Neutrophil-lymphocyte Ratio Predicts Serious Renal Insufficiency in Patients with Lupus Nephritis. Iran. J. Immunol. 2022, 19, 49–57. [Google Scholar]
- Fonseca, J.A.; Gameiro, J.; Duarte, I.; Jorge, S.; Lopes, J.A. The neutrophil-to-lymphocyte ratio as a marker of vasculitis activity, severe infection and mortality in anca-associated vasculitis: A retrospective study. Nefrologia 2021, 41, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, S.; Zhang, G.; Xiong, R.; Li, H. High neutrophil-to-lymphocyte ratio is a significant predictor of cardiovascular and all-cause mortality in patients undergoing peritoneal dialysis. Kidney Blood Press. Res. 2018, 43, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Wang, J.; Peng, Z.; Zhou, Q.; Xiao, X.; Xie, Y.; Wang, W.; Huang, L.; Tang, W.; Sun, D. Neutrophil-to-lymphocyte ratio and incident end-stage renal disease in Chinese patients with chronic kidney disease: Results from the Chinese Cohort Study of Chronic Kidney Disease (C-STRIDE). J. Transl. Med. 2019, 17, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, J.; Wei, D.; Sun, C.; Yang, Y.; Wei, Y.; Liu, X. Prognostic value of the combination of neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio and platelet-to-lymphocyte ratio on mortality in patients on maintenance hemodialysis. MC Nephrol. 2022, 23, 393. [Google Scholar] [CrossRef]
- Zahorec, R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl. Lek. Listy 2021, 122, 474–488. [Google Scholar] [CrossRef]
- Okyay, G.U.; İnal, S.; Öneç, K.; Er, R.E.; Paşaoğlu, Ö.; Paşaoğlu, H.; Derici, Ü.; Erten, Y. Neutrophil to lymphocyte ratio in evaluation of inflammation in patients with chronic kidney disease. Ren. Fail. 2013, 35, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Lippi, G.; Targher, G.; Montagnana, M.; Salvagno, G.L.; Zoppini, G.; Guidi, G.C. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch. Pathol. Lab. Med. 2009, 133, 628–632. [Google Scholar] [CrossRef]


| All Patients (n = 158) | HF/CVD (n = 81) | No HF no CVD (n = 77) | p | |
|---|---|---|---|---|
| RDW (%) | 14.4 (11.4–39.7) | 14.6 (11.7–28.0) | 14.3 (11.4–39.7) | 0.05 |
| Hemoglobin (g/dL) | 12.0 ± 1.7 | 11.8 ± 1.8 | 12.3 ± 1.6 | 0.022 |
| WBC (109/L) | 7.86 (2.17–16.72) | 8.235 (3.76–16.72) | 7.40 (2.17–14.67) | 0.027 |
| NEUT (109/L) | 5.30 (1.0–15.6) | 5.69 (2.25–15.6) | 4.7 (1.0–10.5) | 0.005 |
| LYMPH (109/L) | 1.45 (0.49–4.09) | 1.285 (0.49–4.09) | 1.59 (0.64–3.95) | 0.042 |
| NLR | 3.3 (0.7–23.3) | 3.7 (1.3–23.3) | 2.8 (0.7–9.0) | 0.001 |
| Dp-ucMGP (pM) | 958 (298–5470) | 1024 (351–5000) | 918 (298–5470) | 0.009 |
| Age (years) | 68.5 (25–89) | 71 (33–89) | 62 (25–89) | <0.0001 |
| Gender, Male (%) | 69.0 | 77.8 | 59.7 | 0.011 |
| Hypertension (yes,%) | 76.6 | 74.0 | 79.2 | 0.28 |
| Duration of HT (years) | 11.5 ±10.3 | 12.6 ±11.1 | 10.3 ±9.3 | 0.25 |
| T2DM (yes,%) | 40.5 | 54.3 | 26.0 | <0.0001 |
| Duration of T2DM (years) | 5.7 ± 8.5 | 7.9 ± 9.3 | 3.4 ± 7.0 | <0.0001 |
| BMI (kg/m2) | 26.4 (18.9–43.8) | 26.9 (19–43.8) | 26.1 (18.9–42.2) | 0.15 |
| SBP (mm Hg) | 134.1 ± 22.1 | 131.9 ± 23.8 | 136.4 ± 20.0 | 0.19 |
| DBP (mm Hg) | 82.1 ± 13.1 | 79.5 ± 13.1 | 84.8 ± 12.6 | 0.005 |
| Mean BP (mm Hg) | 106.0 ± 15.8 | 103.5 ± 16.6 | 108.6 ± 14.7 | 0.03 |
| Peripheral pulse pressure | 48 (16–97) | 48 (16–97) | 48 (27–92) | 0.93 |
| Cardiac Rythm | 74.6 ± 13.5 | 72.6 ± 11.6 | 76.8 ± 15.0 | 0.09 |
| Central SBP (mm Hg) | 122.3 ± 20.3 | 119.8 ± 21.3 | 125.0 ± 19.0 | 0.09 |
| Central DBP (mm Hg) | 83.7 ± 13.2 | 80.7 ± 13.4 | 86.7 ± 12.3 | 0.002 |
| Central pulse pressure | 36 (12–79) | 37 (12–77) | 36 (18–79) | 0.78 |
| AI | 23.3 ± 10.4 | 23.3 ± 10.4 | 23.3 ± 10.5 | 0.14 |
| PWV | 8.2 (5.8–12.1) | 8.7 (6.2–12.1) | 8 (5.8–12.0) | 0.24 |
| EGFR (ml/min/1.73m2) | 36.4 ± 20.2 | 31 ± 15 | 41.5 ± 23.2 | 0.05 |
| Calcium (mg/dL) | 8.9 ± 0.8 | 8.9 ± 0.7 | 8.9 ± 0.8 | 0.79 |
| Phosphorus (mg/dL) | 4.2 (2.3–11) | 4.2 (2.4–11) | 4.2 (2.3–8.4) | 0.89 |
| Parathormone (pg/mL) | 131 (7.2–892) | 120 (10–892) | 139 (7.2–820) | 0.76 |
| Albumin (g/dL) | 4.1 (2.4–6.8) | 4.1 (2.4–5.0) | 4.1 (2.6–6.8) | 0.18 |
| Total cholesterol (mg/dL) | 156.4 ± 44.0 | 153.9 ± 43.5 | 159.0 ± 44.8 | 0.52 |
| LDL-cholesterol (mg/dL) | 81.7 ± 38.0 | 80.4 ± 37.8 | 83.0 ± 38.3 | 0.73 |
| HDL-cholesterol (mg/dL) | 44.3 ± 17.7 | 40.6 ± 13.5 | 48.2 ±20.6 | 0.02 |
| Triglycerides (mg/dl) | 138 (17–402) | 140 (17–402) | 134 (48–342) | 0.79 |
| HbA1c (%) | 5.6 (3–10.7) | 5.9 (4.2–9.6) | 5.3 (3–10.7) | <0.0001 |
| CRP (mg/dL) | 0.52 (0.1–77.0) | 0.52 (4.2–9.6) | 0.50 (0.1–77) | 0.27 |
| UACR (mg/g) | 30 (0–5800) | 30 (0–2900) | 20 (0–5800) | 0.64 |
| Warfarin treatment (yes, n) | 8 | 6 | 2 | 0.16 |
| Treatment with ESA (yes, n) | 76 | 41 | 35 | 0.31 |
| RDW | NLR | |||
|---|---|---|---|---|
| Parameters | r | p | r | p |
| Anthropometric parameters | ||||
| Age (years) | 0.12 | 0.15 | 0.19 | 0.02 |
| HT duration (years) | 0.05 | 0.52 | 0.08 | 0.31 |
| T2DM duration (years) | −0.05 | 0.96 | 0.04 | 0.67 |
| CVD duration (years) | 0.15 | 0.07 | 0.32 | <0.0001 |
| SBP (mm Hg) | −0.10 | 0.23 | −0.14 | 0.08 |
| DBP (mm Hg) | −0.19 | 0.02 | −0.3 | <0.0001 |
| Mean BP (mm Hg) | −0.16 | 0.052 | −0.24 | 0.003 |
| BMI (kg/m2) | −0.01 | 0.89 | −0.04 | 0.61 |
| Hematologic parameters from total blood count | ||||
| RDW (%) | - | - | 0.23 | 0.004 |
| NLR | 0.23 | 0.004 | - | - |
| Hemoglobin (g/dL) | −0.36 | <0.0001 | −0.37 | <0.0001 |
| WBC (109/L) | 0.17 | 0.04 | 0.34 | <0.0001 |
| Nutrition–inflammation markers | ||||
| Albumin (g/dL) | −0.24 | 0.002 | −0.29 | <0.0001 |
| Total chol (mg/dL) | −0.15 | 0.06 | 0.008 | 0.92 |
| LDL chol (mg/dL) | −0.08 | 0.33 | 0.09 | 0.25 |
| HDL chol (mg/dL) | −0.14 | 0.09 | −0.07 | 0.40 |
| Triglycerides (mg/dL) | 0.02 | 0.85 | 0.05 | 0.51 |
| HbA1c (%) | −0.04 | 0.61 | 0.05 | 0.57 |
| CRP (mg/dL) | 0.29 | <0.001 | 0.27 | 0.01 |
| CKD markers | ||||
| eGFR (mL/min/1.73 m2) | −0.22 | 0.08 | −0.25 | 0.04 |
| UACR (mg/g) | −0.17 | 0.03 | −0.06 | 0.47 |
| Markers of vascular dysfunction | ||||
| Dp-ucMGP (pM) | 0.43 | <0.0001 | 0.43 | <0.0001 |
| Calcium (mg/dL) | −0.23 | 0.004 | −0.23 | 0.005 |
| Phosphorus (mg/dL) | 0.27 | 0.001 | 0.11 | 0.17 |
| Parathormone (pg/mL) | 0.14 | 0.10 | 0.18 | 0.024 |
| PWV | −0.01 | 0.94 | −0.05 | 0.57 |
| Peripheral pulse pressure | 0.01 | 0.87 | 0.02 | 0.86 |
| Cardiac rhythm (bpm) | −0.16 | 0.05 | −0.14 | 0.08 |
| Central SBP (mm Hg) | −0.10 | 0.23 | −0.15 | 0.06 |
| Central DBP (mm Hg) | −0.19 | 0.02 | −0.32 | <0.0001 |
| Central pulse pressure | 0.03 | 0.72 | 0.07 | 0.42 |
| AI | 0.13 | 0.11 | 0.05 | 0.56 |
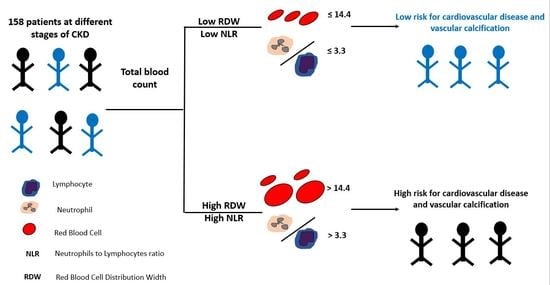
| Quartile 1 NLR ≤ 3.3 and RDW ≤ 14.4 (n = 45) | Quartile 2 NLR ≤ 3.3 and RDW > 14.4 (n = 36) | Quartile 3 NLR > 3.3 and RDW ≤ 14.4 (n = 32) | Quartile 4 NLR > 3.3 and RDW > 14.4 (n = 45) | p | |
|---|---|---|---|---|---|
| RDW (%) | 13.3 (11.4–14.3) | 15.8 (14.4–39.7) | 13.4 (12.3–14.2) | 16.0 (14.4–27.9) | <0.0001 |
| NLR | 2.1 (0.7–3.3) | 2.2 (1.2–6.9) | 5.0 (3.3–14.3) | 5.6 (3.4–23.3) | <0.0001 |
| Hemoglobin (g/dL) | 12.8 ± 1.4 | 12.0 ± 1.7 | 12.1 ± 1.9 | 11.1 ± 1.2 | <0.0001 |
| Dp-ucMGP (pM) | 800.5 (298–4814) | 934 (684–5470) | 1015 (566–4090) | 1442 (658–5000) | <0.0001 |
| CVD (yes, %) | 26.7% | 27.7% | 44.8% | 57.8% | 0.008 |
| Duration of CVD (years) | 2.7 ±5.9 | 2.1 ±4.6 | 3.3 ±4.3 | 4.9 ±6.4 | 0.02 |
| DBP (mm Hg) | 84.9 ± 11.5 | 84.2 ± 12.0 | 83.6 ± 11.9 | 75.4 ± 12.5 | 0.001 |
| Mean BP (mm Hg) | 108.6 ± 14.7 | 108.1 ± 14.8 | 107.2 ± 16.5 | 99.6 ± 15.0 | 0.022 |
| Central DBP (mm Hg) | 86.6 ± 11.1 | 86.4 ± 11.6 | 85 ± 12.0 | 76.5 ± 12.7 | <0.0001 |
| Calcium (mg/dL) | 9.2 ± 0.6 | 9.0 ± 0.8 | 9.0 ± 0.7 | 8.6 ± 0.8 | 0.001 |
| Phosphorus (mg/dL) | 3.7 (2.4–6.5) | 4.5 (2.5–9.4) | 4.1 (2.7–11.0) | 4.5 (2.3–7.6) | 0.01 |
| Albumin (g/dL) | 4.2 (3.4–6.8) | 4.1 (3.2–4.9) | 4.0 (2.9–5.0) | 3.9 (2.4–4.5) | <0.0001 |
| CRP (mg/dL) | 0.3 (0.1–15.0) | 0.6 (0.1–77.0) | 0.5 (0.1–7.7) | 0.8 (0.2–23.3) | <0.0001 |
| UACR (mg/g) | 263 ±614 | 198 ±550 | 550 ±1220 | 191 ±0.612 | 0.04 |
| Use of ESA | 37.7% | 50% | 31% | 71.1% | 0.002 |
| β | SE | p | CI | |
|---|---|---|---|---|
| Model 1 (Unadjusted) | ||||
| Dp-ucMGP | 0.001 | <0.0001 | 0.001 | 0.00–0.002 |
| Model 2 (Adjusted) | ||||
| Dp-ucMGP | 0.001 | <0.0001 | 0.001 | 0.00–0.002 |
| β | SE | p | CI | |
|---|---|---|---|---|
| Model 1 (Unadjusted) | ||||
| Dp-ucMGP | 0.002 | 0.001 | 0.002 | 0.001–0.003 |
| Model 2 (Adjusted) | ||||
| Dp-ucMGP | 0.002 | <0.001 | 0.002 | 0.001–0.003 |
| Mean BP | −0.059 | 0.019 | 0.003 | −0.097 to −0.021 |
| Sex | 1.70 | 0.60 | 0.006 | 0.50–2.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roumeliotis, S.; Neofytou, I.E.; Maassen, C.; Lux, P.; Kantartzi, K.; Papachristou, E.; Schurgers, L.J.; Liakopoulos, V. Association of Red Blood Cell Distribution Width and Neutrophil-to-Lymphocyte Ratio with Calcification and Cardiovascular Markers in Chronic Kidney Disease. Metabolites 2023, 13, 303. https://doi.org/10.3390/metabo13020303
Roumeliotis S, Neofytou IE, Maassen C, Lux P, Kantartzi K, Papachristou E, Schurgers LJ, Liakopoulos V. Association of Red Blood Cell Distribution Width and Neutrophil-to-Lymphocyte Ratio with Calcification and Cardiovascular Markers in Chronic Kidney Disease. Metabolites. 2023; 13(2):303. https://doi.org/10.3390/metabo13020303
Chicago/Turabian StyleRoumeliotis, Stefanos, Ioannis E. Neofytou, Cecile Maassen, Petra Lux, Konstantia Kantartzi, Evangelos Papachristou, Leon J. Schurgers, and Vassilios Liakopoulos. 2023. "Association of Red Blood Cell Distribution Width and Neutrophil-to-Lymphocyte Ratio with Calcification and Cardiovascular Markers in Chronic Kidney Disease" Metabolites 13, no. 2: 303. https://doi.org/10.3390/metabo13020303
APA StyleRoumeliotis, S., Neofytou, I. E., Maassen, C., Lux, P., Kantartzi, K., Papachristou, E., Schurgers, L. J., & Liakopoulos, V. (2023). Association of Red Blood Cell Distribution Width and Neutrophil-to-Lymphocyte Ratio with Calcification and Cardiovascular Markers in Chronic Kidney Disease. Metabolites, 13(2), 303. https://doi.org/10.3390/metabo13020303