Corinthian Currants Supplementation Restores Serum Polar Phenolic Compounds, Reduces IL-1beta, and Exerts Beneficial Effects on Gut Microbiota in the Streptozotocin-Induced Type-1 Diabetic Rat
Abstract
:1. Introduction
2. Materials and Methods
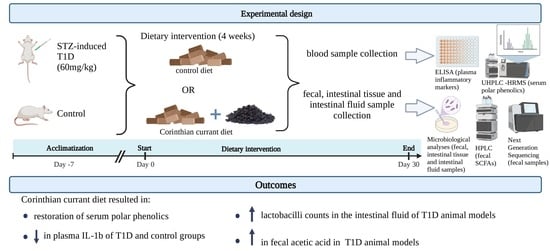
2.1. Animals and Induction of Diabetes
2.2. Dietary Treatment
2.3. Sample Collection
2.4. Blood Analyses
2.4.1. Biochemical Parameters, Insulin, and Inflammatory Factors
2.4.2. Determination of Polar Phenols in Rat Serum
Polar Phenol Isolation from Serum Samples
UHPLC–HRMS Analysis
2.5. Analysis of Fecal Microbiota
2.6. Analysis of Intestinal Tissue Adherent and Intestinal Fluid Microbiota
2.7. DNA Extraction, PCR Amplification, and 16S rRNA Sequencing
2.8. SCFAs
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effect of Corinthian Currants on Body Weight, Biochemical Parameters, Insulin, and Inflammatory Markers
3.2. Polar Phenolics Detected in Rat Serum
3.3. Analysis of the Fecal Microbiota
3.4. Analysis of the Intestinal Tissue and Fluid Microbiota
3.5. Fecal Microbiota Determined by Next-Generation DNA Sequencing
3.6. SCFAs
3.7. Potential Correlations between Circulating Inflammatory Markers, Gut Microbiota, and SCFAs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IDF Diabetes Atlas 2021 | IDF Diabetes Atlas. Available online: https://diabetesatlas.org/ (accessed on 5 January 2023).
- Pugliese, A. Insulitis in the Pathogenesis of Type 1 Diabetes. Pediatr. Diabetes 2016, 17 (Suppl. 22), 31–36. [Google Scholar] [CrossRef] [PubMed]
- Siljander, H.; Honkanen, J.; Knip, M. Microbiome and Type 1 Diabetes. EBioMedicine 2019, 46, 512–521. [Google Scholar] [CrossRef] [Green Version]
- Bakay, M.; Pandey, R.; Grant, S.F.A.; Hakonarson, H. The Genetic Contribution to Type 1 Diabetes. Curr. Diab. Rep. 2019, 19, 116. [Google Scholar] [CrossRef]
- Gradisteanu Pircalabioru, G.; Corcionivoschi, N.; Gundogdu, O.; Chifiriuc, M.-C.; Marutescu, L.G.; Ispas, B.; Savu, O. Dysbiosis in the Development of Type I Diabetes and Associated Complications: From Mechanisms to Targeted Gut Microbes Manipulation Therapies. Int. J. Mol. Sci. 2021, 22, 2763. [Google Scholar] [CrossRef] [PubMed]
- Bezirtzoglou, E.; Stavropoulou, E.; Kantartzi, K.; Tsigalou, C.; Voidarou, C.; Mitropoulou, G.; Prapa, I.; Santarmaki, V.; Kompoura, V.; Yanni, A.E.; et al. Maintaining Digestive Health in Diabetes: The Role of the Gut Microbiome and the Challenge of Functional Foods. Microorganisms 2021, 9, 516. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front. Immunol. 2020, 11, 571731. [Google Scholar] [CrossRef]
- Kudelka, M.R.; Stowell, S.R.; Cummings, R.D.; Neish, A.S. Intestinal Epithelial Glycosylation in Homeostasis and Gut Microbiota Interactions in IBD. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 597–617. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut Microbiota from Green Tea Polyphenol-Dosed Mice Improves Intestinal Epithelial Homeostasis and Ameliorates Experimental Colitis. Microbiome 2021, 9, 184. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Scholz, M.; Lomer, M.C.; Ralph, F.S.; Irving, P.M.; Lindsay, J.O.; Fava, F.; Tuohy, K.; Whelan, K. Gut Microbiota Associations with Diet in Irritable Bowel Syndrome and the Effect of Low FODMAP Diet and Probiotics. Clin. Nutr. Edinb. Scotl. 2021, 40, 1861–1870. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M. Interactions between Gut Microbiota and Host Metabolism Predisposing to Obesity and Diabetes. Annu. Rev. Med. 2011, 62, 361–380. [Google Scholar] [CrossRef]
- Meng, C.; Bai, C.; Brown, T.D.; Hood, L.E.; Tian, Q. Human Gut Microbiota and Gastrointestinal Cancer. Genom. Proteom. Bioinform. 2018, 16, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, X.; Feng, W.; Liu, Q.; Zhou, S.; Liu, Q.; Cai, L. The Gut Microbiota and Its Interactions with Cardiovascular Disease. Microb. Biotechnol. 2020, 13, 637–656. [Google Scholar] [CrossRef] [Green Version]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The Gut Microbiota-Brain Axis in Behaviour and Brain Disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liao, J.; Xia, Y.; Liu, X.; Jones, R.; Haran, J.; McCormick, B.; Sampson, T.R.; Alam, A.; Ye, K. Gut Microbiota Regulate Alzheimer’s Disease Pathologies and Cognitive Disorders via PUFA-Associated Neuroinflammation. Gut 2022, 71, 2233–2252. [Google Scholar] [CrossRef]
- Hamilton-Williams, E.E.; Lorca, G.L.; Norris, J.M.; Dunne, J.L. A Triple Threat? The Role of Diet, Nutrition, and the Microbiota in T1D Pathogenesis. Front. Nutr. 2021, 8, 600756. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Pittayanon, R.; Lau, J.T.; Yuan, Y.; Leontiadis, G.I.; Tse, F.; Surette, M.; Moayyedi, P. Gut Microbiota in Patients With Irritable Bowel Syndrome-A Systematic Review. Gastroenterology 2019, 157, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Johansson, M.A.; Björkander, S.; Mata Forsberg, M.; Qazi, K.R.; Salvany Celades, M.; Bittmann, J.; Eberl, M.; Sverremark-Ekström, E. Probiotic Lactobacilli Modulate Staphylococcus Aureus-Induced Activation of Conventional and Unconventional T Cells and NK Cells. Front. Immunol. 2016, 7, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzello, V.; Bonaccorsi, I.; Dongarrà, M.L.; Fink, L.N.; Ferlazzo, G. Role of Natural Killer and Dendritic Cell Crosstalk in Immunomodulation by Commensal Bacteria Probiotics. J. Biomed. Biotechnol. 2011, 2011, 473097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dedrick, S.; Sundaresh, B.; Huang, Q.; Brady, C.; Yoo, T.; Cronin, C.; Rudnicki, C.; Flood, M.; Momeni, B.; Ludvigsson, J.; et al. The Role of Gut Microbiota and Environmental Factors in Type 1 Diabetes Pathogenesis. Front. Endocrinol. 2020, 11, 78. [Google Scholar] [CrossRef]
- Xiao, J. Recent Advances in Dietary Flavonoids for Management of Type 2 Diabetes. Curr. Opin. Food Sci. 2022, 44, 100806. [Google Scholar] [CrossRef]
- Li, B.; Pan, L.-L.; Pan, X.; Dong, X.; Ren, Z.; Zhang, H.; Chen, W.; de Vos, P.; Sun, J. Opportunities and Challenges of Polyphenols and Polysaccharides for Type 1 Diabetes Intervention. Crit. Rev. Food Sci. Nutr. 2022, 1–13. [Google Scholar] [CrossRef]
- Hurst, R.D.; Lyall, K.A.; Wells, R.W.; Sawyer, G.M.; Lomiwes, D.; Ngametua, N.; Hurst, S.M. Daily Consumption of an Anthocyanin-Rich Extract Made From New Zealand Blackcurrants for 5 Weeks Supports Exercise Recovery Through the Management of Oxidative Stress and Inflammation: A Randomized Placebo Controlled Pilot Study. Front. Nutr. 2020, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Castro-Acosta, M.L.; Smith, L.; Miller, R.J.; McCarthy, D.I.; Farrimond, J.A.; Hall, W.L. Drinks Containing Anthocyanin-Rich Blackcurrant Extract Decrease Postprandial Blood Glucose, Insulin and Incretin Concentrations. J. Nutr. Biochem. 2016, 38, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Cook, M.D.; Myers, S.D.; Gault, M.L.; Edwards, V.C.; Willems, M.E.T. Cardiovascular Function during Supine Rest in Endurance-Trained Males with New Zealand Blackcurrant: A Dose-Response Study. Eur. J. Appl. Physiol. 2017, 117, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Guo, J.; Yin, M.; Liu, Y.; You, Y.; Zhan, J.; Huang, W. Grape Extract Activates Brown Adipose Tissue Through Pathway Involving the Regulation of Gut Microbiota and Bile Acid. Mol. Nutr. Food Res. 2020, 64, 2000149. [Google Scholar] [CrossRef]
- Chiou, A.; Karathanos, V.T.; Mylona, A.; Salta, F.N.; Preventi, F.; Andrikopoulos, N.K. Currants (Vitis Vinifera L.) Content of Simple Phenolics and Antioxidant Activity. Food Chem. 2007, 102, 516–522. [Google Scholar] [CrossRef]
- Chiou, A.; Panagopoulou, E.A.; Gatzali, F.; De Marchi, S.; Karathanos, V.T. Anthocyanins Content and Antioxidant Capacity of Corinthian Currants (Vitis Vinifera L., Var. Apyrena). Food Chem. 2014, 146, 157–165. [Google Scholar] [CrossRef]
- Kanellos, P.T.; Kaliora, A.C.; Gioxari, A.; Christopoulou, G.O.; Kalogeropoulos, N.; Karathanos, V.T. Absorption and Bioavailability of Antioxidant Phytochemicals and Increase of Serum Oxidation Resistance in Healthy Subjects Following Supplementation with Raisins. Plant Foods Hum. Nutr. Dordr. Neth. 2013, 68, 411–415. [Google Scholar] [CrossRef]
- Vasilakopoulou, P.B.; Gousgouni, A.-T.; Yanni, A.E.; Kostomitsopoulos, N.; Karathanos, V.T.; Chiou, A. Polar Phenol Detection in Plasma and Serum: Insights on Sample Pre-Treatment for LC/MS Analysis and Application on the Serum of Corinthian Currant-Fed Rats. Biomolecules 2022, 12, 1838. [Google Scholar] [CrossRef]
- Kanellos, P.T.; Kaliora, A.C.; Liaskos, C.; Tentolouris, N.K.; Perrea, D.; Karathanos, V.T. A Study of Glycemic Response to Corinthian Raisins in Healthy Subjects and in Type 2 Diabetes Mellitus Patients. Plant Foods Hum. Nutr. Dordr. Neth. 2013, 68, 145–148. [Google Scholar] [CrossRef]
- Yanni, A.E.; Efthymiou, V.; Lelovas, P.; Agrogiannis, G.; Kostomitsopoulos, N.; Karathanos, V.T. Effects of Dietary Corinthian Currants (Vitis Vinifera L., Var. Apyrena) on Atherosclerosis and Plasma Phenolic Compounds during Prolonged Hypercholesterolemia in New Zealand White Rabbits. Food Funct. 2015, 6, 963–971. [Google Scholar] [CrossRef]
- AL-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- Kawser Hossain, M.; Abdal Dayem, A.; Han, J.; Yin, Y.; Kim, K.; Kumar Saha, S.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. Molecular Mechanisms of the Anti-Obesity and Anti-Diabetic Properties of Flavonoids. Int. J. Mol. Sci. 2016, 17, 569. [Google Scholar] [CrossRef] [Green Version]
- Nasri, S.; Roghani, M.; Baluchnejadmojarad, T.; Rabani, T.; Balvardi, M. Vascular Mechanisms of Cyanidin-3-Glucoside Response in Streptozotocin-Diabetic Rats. Pathophysiol. Off. J. Int. Soc. Pathophysiol. 2011, 18, 273–278. [Google Scholar] [CrossRef]
- Zhu, W.; Jia, Q.; Wang, Y.; Zhang, Y.; Xia, M. The Anthocyanin Cyanidin-3-O-β-Glucoside, a Flavonoid, Increases Hepatic Glutathione Synthesis and Protects Hepatocytes against Reactive Oxygen Species during Hyperglycemia: Involvement of a CAMP-PKA-Dependent Signaling Pathway. Free Radic. Biol. Med. 2012, 52, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Daveri, E.; Cremonini, E.; Mastaloudis, A.; Hester, S.N.; Wood, S.M.; Waterhouse, A.L.; Anderson, M.; Fraga, C.G.; Oteiza, P.I. Cyanidin and Delphinidin Modulate Inflammation and Altered Redox Signaling Improving Insulin Resistance in High Fat-Fed Mice. Redox Biol. 2018, 18, 16–24. [Google Scholar] [CrossRef]
- Tanveer, A.; Akram, K.; Farooq, U.; Hayat, Z.; Shafi, A. Management of Diabetic Complications through Fruit Flavonoids as a Natural Remedy. Crit. Rev. Food Sci. Nutr. 2017, 57, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Morand, C.; Manach, C.; Rémésy, C. Absorption and Metabolism of Polyphenols in the Gut and Impact on Health. Biomed. Pharmacother. Biomed. Pharmacother. 2002, 56, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H. Microbiota or Short-Chain Fatty Acids: Which Regulates Diabetes? Cell. Mol. Immunol. 2018, 15, 88–91. [Google Scholar] [CrossRef] [Green Version]
- Müller, M.; Gonzalez Hernandez, M.A.; Goossens, G.; Reijnders, D.; Holst, J.; Jocken, J.; Eijk, H.; Canfora, E.; Blaak, E. Circulating but Not Faecal Short-Chain Fatty Acids Are Related to Insulin Sensitivity, Lipolysis and GLP-1 Concentrations in Humans. Sci. Rep. 2019, 9, 12515. [Google Scholar] [CrossRef] [Green Version]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The Short-Chain Fatty Acid Acetate Reduces Appetite via a Central Homeostatic Mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-Chain Fatty Acids Activate GPR41 and GPR43 on Intestinal Epithelial Cells to Promote Inflammatory Responses in Mice. Gastroenterology 2013, 145, 396–406.e1–10. [Google Scholar] [CrossRef]
- Yanni, A.E.; Mitropoulou, G.; Prapa, I.; Agrogiannis, G.; Kostomitsopoulos, N.; Bezirtzoglou, E.; Kourkoutas, Y.; Karathanos, V.T. Functional Modulation of Gut Microbiota in Diabetic Rats Following Dietary Intervention with Pistachio Nuts (Pistacia Vera L.). Metab. Open 2020, 7, 100040. [Google Scholar] [CrossRef]
- Prapa, I.; Yanni, A.E.; Nikolaou, A.; Kostomitsopoulos, N.; Kalogeropoulos, N.; Bezirtzoglou, E.; Karathanos, V.T.; Kourkoutas, Y. Dietary Pistachio (Pistacia Vera L.) Beneficially Alters Fatty Acid Profiles in Streptozotocin-Induced Diabetic Rat. Appl. Sci. 2022, 12, 4606. [Google Scholar] [CrossRef]
- Morrow, T.J. Animal Models of Painful Diabetic Neuropathy: The STZ Rat Model. Curr. Protoc. Neurosci. 2004, 29, 9.18.1–9.18.11. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5.47.1–5.47.20. [Google Scholar] [CrossRef] [PubMed]
- Koh, G.Y.; Rowling, M.J.; Schalinske, K.L.; Grapentine, K.; Loo, Y.T. Consumption of Dietary Resistant Starch Partially Corrected the Growth Pattern Despite Hyperglycemia and Compromised Kidney Function in Streptozotocin-Induced Diabetic Rats. J. Agric. Food Chem. 2016, 64, 7540–7545. [Google Scholar] [CrossRef]
- Yadav, H.; Jain, S.; Sinha, P.R. Oral Administration of Dahi Containing Probiotic Lactobacillus Acidophilus and Lactobacillus Casei Delayed the Progression of Streptozotocin-Induced Diabetes in Rats. J. Dairy Res. 2008, 75, 189–195. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasilakopoulou, P.B.; Fanarioti, Ε.; Tsarouchi, M.; Kokotou, M.G.; Dermon, C.R.; Karathanos, V.T.; Chiou, A. Polar Phenol Detection in Rat Brain: Development and Validation of a Versatile UHPLC-MS Method and Application on the Brain Tissues of Corinthian Currant (Vitis Vinifera L.,Var. Apyrena) Fed Rats. Food Chem. 2022, 390, 133131. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Li, S.; Wen, Y.; Luo, Y.; Huang, J.; Chen, B.; Lv, S.; Chen, L.; He, L.; He, M.; et al. Characteristics of Intestinal Microbiota in C57BL/6 Mice with Non-Alcoholic Fatty Liver Induced by High-Fat Diet. Front. Microbiol. 2022, 13, 1051200. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Deng, X.; Yang, X.; Wang, J.; Li, T.; Hua, G.; Han, D.; Da, L.; Li, R.; Rong, W.; et al. Characteristics of Bacterial Microbiota in Different Intestinal Segments of Aohan Fine-Wool Sheep. Front. Microbiol. 2022, 13, 874536. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Reitmeier, S.; Kumar, N.; Clavel, T. Rhea: A Transparent and Modular R Pipeline for Microbial Profiling Based on 16S RRNA Gene Amplicons. PeerJ 2017, 5, e2836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolaou, A.; Galanis, A.; Kanellaki, M.; Tassou, C.; Akrida-Demertzi, K.; Kourkoutas, Y. Assessment of Free and Immobilized Kefir Culture in Simultaneous Alcoholic and Malolactic Cider Fermentations. LWT—Food Sci. Technol. 2017, 76, 67–78. [Google Scholar] [CrossRef]
- Huda-Faujan, N.; Abdulamir, A.S.; Fatimah, A.B.; Anas, O.M.; Shuhaimi, M.; Yazid, A.M.; Loong, Y.Y. The Impact of the Level of the Intestinal Short Chain Fatty Acids in Inflammatory Bowel Disease Patients versus Healthy Subjects. Open Biochem. J. 2010, 4, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Hossein-Nia, B.; Khorram, S.; Rezazadeh, H.; Safaiyan, A.; Ghiasi, R.; Tarighat-Esfanjani, A. The Effects of Natural Clinoptilolite and Nano-Sized Clinoptilolite Supplementation on Lipid Profile, Food Intakes and Body Weight in Rats with Streptozotocin-Induced Diabetes. Adv. Pharm. Bull. 2018, 8, 211–216. [Google Scholar] [CrossRef]
- Jing, S.; Zhao, Z.; Wu, J.; Yan, L.-J. Antioxidative and Hypoglycemic Effect of Ta-Ermi Extracts on Streptozotocin-Induced Diabetes. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 2147–2155. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, Y.; Ozeki, A.; Tani, T.; Tsuda, T. Blackcurrant Extract Ameliorates Hyperglycemia in Type 2 Diabetic Mice in Association with Increased Basal Secretion of Glucagon-Like Peptide-1 and Activation of AMP-Activated Protein Kinase. J. Nutr. Sci. Vitaminol. 2018, 64, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Cano-Cano, F.; Gómez-Jaramillo, L.; Ramos-García, P.; Arroba, A.I.; Aguilar-Diosdado, M. IL-1β Implications in Type 1 Diabetes Mellitus Progression: Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1303. [Google Scholar] [CrossRef]
- Giribabu, N.; Karim, K.; Kilari, E.K.; Kassim, N.M.; Salleh, N. Anti-Inflammatory, Antiapoptotic and Proproliferative Effects of Vitis Vinifera Seed Ethanolic Extract in the Liver of Streptozotocin-Nicotinamide-Induced Type 2 Diabetes in Male Rats. Can. J. Diabetes 2018, 42, 138–149. [Google Scholar] [CrossRef]
- Chang, C.-C.; Chang, C.-Y.; Huang, J.-P.; Hung, L.-M. Effect of Resveratrol on Oxidative and Inflammatory Stress in Liver and Spleen of Streptozotocin-Induced Type 1 Diabetic Rats. Chin. J. Physiol. 2012, 55, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Benn, T.; Kim, B.; Park, Y.-K.; Wegner, C.J.; Harness, E.; Nam, T.-G.; Kim, D.-O.; Lee, J.S.; Lee, J.-Y. Polyphenol-Rich Blackcurrant Extract Prevents Inflammation in Diet-Induced Obese Mice. J. Nutr. Biochem. 2014, 25, 1019–1025. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, B.; Yang, Y.; Pham, T.X.; Park, Y.-K.; Manatou, J.; Koo, S.I.; Chun, O.K.; Lee, J.-Y. Berry Anthocyanins Suppress the Expression and Secretion of Proinflammatory Mediators in Macrophages by Inhibiting Nuclear Translocation of NF-ΚB Independent of NRF2-Mediated Mechanism. J. Nutr. Biochem. 2014, 25, 404–411. [Google Scholar] [CrossRef]
- Sepehr, E.; Cooke, G.; Robertson, P.; Gilani, G.S. Bioavailability of Soy Isoflavones in Rats Part I: Application of Accurate Methodology for Studying the Effects of Gender and Source of Isoflavones. Mol. Nutr. Food Res. 2007, 51, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Passon, M. Dietary Phenolic Compounds in Biological Samples: Current Challenges in Analytical Chemistry. In Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-319-78030-6. [Google Scholar]
- Taylor, J.I.; Grace, P.B.; Bingham, S.A. Optimization of Conditions for the Enzymatic Hydrolysis of Phytoestrogen Conjugates in Urine and Plasma. Anal. Biochem. 2005, 341, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Hu, X.; Qiao, M.; Li, Y.; Cao, S.; Ding, L.; Feng, X.; Kang, N.; Zhang, D.; Qiu, F. Simultaneous Determination of Five Isoflavones in Rat Plasma by LC-MS/MS: Comparative Pharmacokinetic Characteristics of Puerariae Lobatae Radix in Normal and Type 2 Diabetic Rats. J. Sep. Sci. 2019, 42, 2592–2601. [Google Scholar] [CrossRef]
- Yao, Y.; Ma, X.; Li, T.; Guo, H.; Chang, R.; Liu, J.; Liu, Q.; Hao, H.; Huang, T.; Chen, W.; et al. Quantification of Isoflavone Glycosides and Aglycones in Rat Plasma by LC-MS/MS: Troubleshooting of Interference from Food and Its Application to Pharmacokinetic Study of Semen Sojae Praeparatum Extract. J. Pharm. Biomed. Anal. 2018, 161, 444–454. [Google Scholar] [CrossRef]
- Gilani, G.S.; Farmer, C.; Dyck, M.; Robertson, P.; Dahiya, J.; Sepehr, E.; Fan, L.; Nicolidakis, H.; Curran, I.; Cooke, G.M. Distribution of Isoflavones in Samples of Serum, Liver and Mammary Glands of Rats or Pigs Fed Dietary Isoflavones. Ann. Nutr. Metab. 2011, 58, 171–180. [Google Scholar] [CrossRef]
- Bustamante, L.; Pastene, E.; Duran-Sandoval, D.; Vergara, C.; Von Baer, D.; Mardones, C. Pharmacokinetics of Low Molecular Weight Phenolic Compounds in Gerbil Plasma after the Consumption of Calafate Berry (Berberis Microphylla) Extract. Food Chem. 2018, 268, 347–354. [Google Scholar] [CrossRef]
- Nie, T.; Cooper, G.J.S. Mechanisms Underlying the Antidiabetic Activities of Polyphenolic Compounds: A Review. Front. Pharmacol. 2021, 12, 798329. [Google Scholar] [CrossRef]
- Deng, Y.-X.; Shi, Q.-Z.; Chen, B.; Zhang, X.-J.; Liu, S.-Z.; Qiu, X.-M. Comparative Pharmacokinetics of Baicalin in Normal and the Type 2 Diabetic Rats after Oral Administration of the Radix Scutellariae Extract. Fitoterapia 2012, 83, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Cai, Z.; Shenghui, L.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A Metagenome-Wide Association Study of Gut Microbiota in Type 2 Diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Chen, R.; Wu, P.; Cai, Z.; Tang, L.; Ye, L.; Hou, C.; Yang, N.; Zhao, J. The Combination of Puerariae Lobatae Radix and Chuanxiong Rhizoma Enhanced the Absorption and Pharmacokinetics of Puerarin by Modulating the Intestinal Barrier and Influenced Gut Microbiota. J. Funct. Foods 2018, 47, 72–82. [Google Scholar] [CrossRef]
- He, M.-Y.; Deng, Y.-X.; Shi, Q.-Z.; Zhang, X.-J.; Lv, Y. Comparative Pharmacokinetic Investigation on Baicalin and Wogonoside in Type 2 Diabetic and Normal Rats after Oral Administration of Traditional Chinese Medicine Huanglian Jiedu Decoction. J. Ethnopharmacol. 2014, 155, 334–342. [Google Scholar] [CrossRef]
- Yan, H.; Lu, J.; Wang, Y.; Gu, W.; Yang, X.; Yu, J. Intake of Total Saponins and Polysaccharides from Polygonatum Kingianum Affects the Gut Microbiota in Diabetic Rats. Phytomed. Int. J. Phytother. Phytopharm. 2017, 26, 45–54. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, C.; Guven, E.C.; Paoli, P.; Simal-Gandara, J.; Ramkumar, K.M.; Wang, S.; Buleu, F.; Pah, A.; Turi, V.; et al. Dietary Polyphenols as Antidiabetic Agents: Advances and Opportunities. Food Front. 2020, 1, 18–44. [Google Scholar] [CrossRef] [Green Version]
- Di Meo, F.; Lemaur, V.; Cornil, J.; Lazzaroni, R.; Duroux, J.-L.; Olivier, Y.; Trouillas, P. Free Radical Scavenging by Natural Polyphenols: Atom versus Electron Transfer. J. Phys. Chem. A 2013, 117, 2082–2092. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free Radicals, Natural Antioxidants, and Their Reaction Mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef] [Green Version]
- Kristich, C.J.; Rice, L.B.; Arias, C.A. Enterococcal Infection—Treatment and Antibiotic Resistance. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Gilmore, M.S., Clewell, D.B., Ike, Y., Shankar, N., Eds.; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Mejía-León, M.E.; López-Domínguez, L.; Aguayo-Patrón, S.V.; Caire-Juvera, G.; Calderón de la Barca, A.M. Dietary Changes and Gut Dysbiosis in Children With Type 1 Diabetes. J. Am. Coll. Nutr. 2018, 37, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Marques, T.M.; O’Sullivan, O.; Fitzgerald, P.; Fitzgerald, G.F.; Cotter, P.D.; Dinan, T.G.; Cryan, J.F.; Stanton, C.; Ross, R.P. Streptozotocin-Induced Type-1-Diabetes Disease Onset in Sprague-Dawley Rats Is Associated with an Altered Intestinal Microbiota Composition and Decreased Diversity. Microbiol. Read. Engl. 2015, 161, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Marteau, P.; Pochart, P.; Doré, J.; Béra-Maillet, C.; Bernalier, A.; Corthier, G. Comparative Study of Bacterial Groups within the Human Cecal and Fecal Microbiota. Appl. Environ. Microbiol. 2001, 67, 4939–4942. [Google Scholar] [CrossRef] [Green Version]
- Ruiz de la Bastida, A.; Peirotén, Á.; Langa, S.; Álvarez, I.; Arqués, J.L.; Landete, J.M. Metabolism of Flavonoids and Lignans by Lactobacilli and Bifidobacteria Strains Improves the Nutritional Properties of Flaxseed-Enriched Beverages. Food Res. Int. 2021, 147, 110488. [Google Scholar] [CrossRef]
- Marras, L.; Caputo, M.; Bisicchia, S.; Soato, M.; Bertolino, G.; Vaccaro, S.; Inturri, R. The Role of Bifidobacteria in Predictive and Preventive Medicine: A Focus on Eczema and Hypercholesterolemia. Microorganisms 2021, 9, 836. [Google Scholar] [CrossRef]
- Mandalari, G.; Chessa, S.; Bisignano, C.; Chan, L.; Carughi, A. The Effect of Sun-Dried Raisins (Vitis Vinifera L.) on the in Vitro Composition of the Gut Microbiota. Food Funct. 2016, 7, 4048–4060. [Google Scholar] [CrossRef]
- Paturi, G.; Butts, C.A.; Monro, J.A.; Hedderley, D. Effects of Blackcurrant and Dietary Fibers on Large Intestinal Health Biomarkers in Rats. Plant Foods Hum. Nutr. Dordr. Neth. 2018, 73, 54–60. [Google Scholar] [CrossRef]
- Slattery, C.; Cotter, P.D.; O’Toole, P.W. Analysis of Health Benefits Conferred by Lactobacillus Species from Kefir. Nutrients 2019, 11, 1252. [Google Scholar] [CrossRef] [Green Version]
- Parks, D.H.; Chuvochina, M.; Waite, D.W.; Rinke, C.; Skarshewski, A.; Chaumeil, P.-A.; Hugenholtz, P. A Standardized Bacterial Taxonomy Based on Genome Phylogeny Substantially Revises the Tree of Life. Nat. Biotechnol. 2018, 36, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Skennerton, C.T.; Haroon, M.F.; Briegel, A.; Shi, J.; Jensen, G.J.; Tyson, G.W.; Orphan, V.J. Phylogenomic Analysis of Candidatus “Izimaplasma” Species: Free-Living Representatives from a Tenericutes Clade Found in Methane Seeps. ISME J. 2016, 10, 2679–2692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, F.-C.; Lian, C.-A.; He, L.-S. Genomic Characterization of a Novel Tenericutes Bacterium from Deep-Sea Holothurian Intestine. Microorganisms 2020, 8, 1874. [Google Scholar] [CrossRef]
- Almeida, A.; Mitchell, A.L.; Boland, M.; Forster, S.C.; Gloor, G.B.; Tarkowska, A.; Lawley, T.D.; Finn, R.D. A New Genomic Blueprint of the Human Gut Microbiota. Nature 2019, 568, 499–504. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Wang, X.; Huang, Y.; Bu, X.; Xiao, S.; Qin, C.; Qiao, F.; Qin, J.G.; Chen, L. Effects of Dietary T-2 Toxin on Gut Health and Gut Microbiota Composition of the Juvenile Chinese Mitten Crab (Eriocheir Sinensis). Fish Shellfish Immunol. 2020, 106, 574–582. [Google Scholar] [CrossRef]
- Brown, C.T.; Davis-Richardson, A.G.; Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; et al. Gut Microbiome Metagenomics Analysis Suggests a Functional Model for the Development of Autoimmunity for Type 1 Diabetes. PLoS ONE 2011, 6, e25792. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Huang, G.; Nagy, T.; Teng, Q.; Guo, T.L. Sex-Dependent Effects of Bisphenol A on Type 1 Diabetes Development in Non-Obese Diabetic (NOD) Mice. Arch. Toxicol. 2019, 93, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Lazarevic, V.; Gaïa, N.; Johansson, M.; Ståhlman, M.; Backhed, F.; Delzenne, N.M.; Schrenzel, J.; François, P.; Cani, P.D. Microbiome of Prebiotic-Treated Mice Reveals Novel Targets Involved in Host Response during Obesity. ISME J. 2014, 8, 2116–2130. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, L.; Xue, J.; Yang, X.; Dong, X.; Sha, L.; Lei, H.; Zhang, X.; Zhu, L.; Wang, Z.; et al. Dietary Inulin Alleviates Diverse Stages of Type 2 Diabetes Mellitus via Anti-Inflammation and Modulating Gut Microbiota in Db/Db Mice. Food Funct. 2019, 10, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, S.; Yang, Y.; Wang, Z.; Wang, B.; Zhang, B.; Yu, J.; Lu, W.; Pan, M.; Zhao, J.; et al. The Diversity of Gut Microbiota in Type 2 Diabetes with or without Cognitive Impairment. Aging Clin. Exp. Res. 2021, 33, 589–601. [Google Scholar] [CrossRef]
- Huang, Z.; Zeng, S.; Xiong, J.; Hou, D.; Zhou, R.; Xing, C.; Wei, D.; Deng, X.; Yu, L.; Wang, H.; et al. Microecological Koch’s Postulates Reveal That Intestinal Microbiota Dysbiosis Contributes to Shrimp White Feces Syndrome. Microbiome 2020, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Alkanani, A.K.; Hara, N.; Gottlieb, P.A.; Ir, D.; Robertson, C.E.; Wagner, B.D.; Frank, D.N.; Zipris, D. Alterations in Intestinal Microbiota Correlate With Susceptibility to Type 1 Diabetes. Diabetes 2015, 64, 3510–3520. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Rigatto, K.; Gazzana, M.B.; Knorst, M.M.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. Altered Gut Microbiome Profile in Patients With Pulmonary Arterial Hypertension. Hypertens. Dallas Tex 1979 2020, 75, 1063–1071. [Google Scholar] [CrossRef]
- Schmidt, T.S.B.; Raes, J.; Bork, P. The Human Gut Microbiome: From Association to Modulation. Cell 2018, 172, 1198–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishino, K.; Nishida, A.; Inoue, R.; Kawada, Y.; Ohno, M.; Sakai, S.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Kawahara, M.; et al. Analysis of Endoscopic Brush Samples Identified Mucosa-Associated Dysbiosis in Inflammatory Bowel Disease. J. Gastroenterol. 2018, 53, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of Inflammatory Responses by Gut Microbiota and Chemoattractant Receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Du, Z.R.; Wang, X.; Sun, X.R.; Zhao, Q.; Zhao, F.; Wong, W.T.; Wong, K.H.; Dong, X.-L. Polymannuronic Acid Prebiotic plus Lacticaseibacillus Rhamnosus GG Probiotic as a Novel Synbiotic Promoted Their Separate Neuroprotection against Parkinson’s Disease. Food Res. Int. Ott. Ont 2022, 155, 111067. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, X.; Li, H.; Li, Y.; Shi, X.; Zhao, F.; Xu, X.; Li, C.; Zhou, G. Intake of Meat Proteins Substantially Increased the Relative Abundance of Genus Lactobacillus in Rat Feces. PLoS ONE 2016, 11, e0152678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Wang, H.; Zhu, M.-J. A Sensitive GC/MS Detection Method for Analyzing Microbial Metabolites Short Chain Fatty Acids in Fecal and Serum Samples. Talanta 2019, 196, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Shao, J.; Luo, Y.; Cai, W.; Chen, L. Rapid and Accurate Simultaneous Determination of Seven Short-Chain Fatty Acids in Feces by Gas Chromatography—Mass Spectrometry (GC-MS): Application in Type 2 Diabetic Rats and Drug Therapy. Anal. Lett. 2020, 53, 2320–2336. [Google Scholar] [CrossRef]
- Zhu, L.; Sha, L.; Li, K.; Wang, Z.; Wang, T.; Li, Y.; Liu, P.; Dong, X.; Dong, Y.; Zhang, X.; et al. Dietary Flaxseed Oil Rich in Omega-3 Suppresses Severity of Type 2 Diabetes Mellitus via Anti-Inflammation and Modulating Gut Microbiota in Rats. Lipids Health Dis. 2020, 19, 20. [Google Scholar] [CrossRef] [Green Version]
- González Hernández, M.A.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liong, M.T.; Shah, N.P. Effects of a Lactobacillus Casei Synbiotic on Serum Lipoprotein, Intestinal Microflora, and Organic Acids in Rats. J. Dairy Sci. 2006, 89, 1390–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinna, C.; Vecchiato, C.; Zaghini, G.; Grandi, M.; Nannoni, E.; Stefanelli, C.; Biagi, G. In Vitro Influence of Dietary Protein and Fructooligosaccharides on Metabolism of Canine Fecal Microbiota. BMC Vet. Res. 2016, 12, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, S.; Guo, W.; Chen, C.; Tang, X.; Zhao, J.; Mao, B.; Zhang, H. Metagenomic Analysis of the Effects of Lactiplantibacillus Plantarum and Fructooligosaccharides (FOS) on the Fecal Microbiota Structure in Mice. Foods 2022, 11, 1187. [Google Scholar] [CrossRef] [PubMed]





| Control Diet | Corinthian Currant Diet | |
|---|---|---|
| Proteins (g) | 18.5 | 17.0 |
| Fats (g) | 3.0 | 2.7 |
| Carbohydrates (g) | 46.3 | 49.0 |
| Dietary fiber (g) | 6.0 | 6.1 |
| Currants (g) | - | 10.0 |
| Energy (Kcal) | 387.6 | 380.8 |
| Polar Phenol (ng/mL Serum) | CD | DCD | CCD | DCCD |
|---|---|---|---|---|
| Flavonols | ||||
| Isorhamnetin | tr | n.d. | 6.45 ± 1.65 a | tr b |
| Kaempferol | 3.37 ± 2.35 | tr a | 8.13 ± 1.6 a | 7.86 ± 0 c |
| Quercetin | 0.57 ± 0.13 | n.d. a | 9.58 ± 3.75 a | 0.66 ± 0.05 b,c |
| Flavones | ||||
| Apigenin | 40.96 ± 10.63 | 0.93 ± 0.44 a | 189.75 ± 40.87 a | 130.8 ± 14.55 b,c |
| Chrysin | n.d. | n.d. | 2.18 ± 0.51 a | tr b |
| Luteolin | 5.56 ± 2.03 | 1.02 ± 0.7 a | 125.69 ± 54.07 a | 77.51 ± 26.82 c |
| Flavanones | ||||
| Hesperetin | 2.13 ± 0.45 | 1.36 ± 1.32 | 10.82 ± 2.63 a | 8.97 ± 1.97 c |
| Naringenin | 3.45 ± 0.48 | 1.78 ± 1.22 a | 9.33 ± 2.18 a | 9.59 ± 3.19 c |
| Isoflavones | ||||
| Daidzein | 5.07 ± 1.32 | 3.3 ± 1.81 | 18.12 ± 5.35 a | 27.51 ± 6.56 b,c |
| Formononetin | 30.93 ± 3.43 | 2.14 ± 1.85 a | 93.35 ± 22.32 a | 66.85 ± 23.27 c |
| Genistein | 14.91 ± 1.69 | 1.25 ± 0.93 a | 42.74 ± 13.91 a | 42.35 ± 11.92 c |
| Benzoic acid derivatives | ||||
| Vanillic acid | 684.37 ± 12.49 | 807.2 ± 88.93 a | 937.59 ± 131.57 a | n.d. b |
| Syringic acid | 13.53 ± 1.18 | 24.78 ± 13.1 | 45.12 ± 8.92 a | 4.08 ± 0.9 b,c |
| Cinnamic acid derivatives | ||||
| Trans-Cinnamic acid | 190.02 ± 81.23 | 167.45 ± 83.02 | 278.22 ± 93.87 | 397 ± 122 c |
| CD | CCD | DCD | DCCD | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 4w | Baseline | 4w | Baseline | 4w | Baseline | 4w | |
| TAC | 8.28 ± 0.41 | 8.44 ± 0.26 | 8.38 ± 0.25 | 8.59 ± 0.36 | 8.26 ± 0.14 | 8.49 ± 0.41 | 8.58 ± 0.21 | 8.59 ± 0.19 |
| Enterococci | 5.98 ± 0.31 | 6.03 ± 0.32 | 6.12 ± 0.36 | 6.04 ± 0.20 | 6.31 ± 0.23 | 6.98 ± 0.33 a,b | 6.50 ± 0.44 | 6.61 ± 0.41 |
| Coliforms | 4.39 ± 0.52 | 4.49 ± 0.34 | 4.85 ± 0.41 | 4.93 ± 0.31 | 5.60 ± 0.65 a,b | 6.80 ± 0.51 a,b | 5.88 ± 0.63 a,b | 7.06 ± 0.43 a,b |
| Enterobacteriaceae | 4.42 ± 0.55 | 4.59 ± 0.35 | 4.90 ± 0.41 | 4.94 ± 0.31 | 5.69 ± 0.62 a,b | 6.85 ± 0.47 a,b | 5.87 ± 0.60 a,b | 7.04 ± 0.44 a,b |
| Staphylococci | 6.28 ± 0.28 | 5.95 ± 0.29 | 6.68 ± 0.28 | 6.29 ± 0.26 | 6.11 ± 0.20 | 7.07 ± 0.32 | 6.48 ± 0.67 | 6.52 ± 0.62 |
| E. coli | 4.33 ± 0.65 | 4.32 ± 0.43 | 4.77 ± 0.44 | 4.78 ± 0.29 | 5.41 ± 0.73 a,b | 6.48 ± 0.53 a,b | 5.86 ± 0.63 a,b | 6.78 ± 0.33 a,b |
| Clostridia | 6.91 ± 0.31 | 6.89 ± 0.54 | 6.75 ± 0.3 | 6.96 ± 0.34 | 7.82 ± 0.27 a,b | 8.27 ± 0.44 a,b | 8.48 ± 0.15 a,b | 8.06 ± 0.20 a,b |
| Bifidobacteria | 8.25 ± 0.14 | 8.25 ± 0.36 | 8.28 ± 0.26 | 8.30 ± 0.32 | 8.25 ± 0.26 | 8.46 ± 0.36 | 8.29 ± 0.29 | 8.79 ± 0.32 |
| Lactobacilli | 8.47 ± 0.36 | 8.69 ± 0.22 | 8.65 ± 0.12 | 8.72 ± 0.26 | 8.36 ± 0.05 | 8.48 ± 0.36 | 8.54 ± 0.28 | 8.56 ± 0.26 |
| CD | CCD | DCD | DCCD | |||||
|---|---|---|---|---|---|---|---|---|
| Intestinal Fluid | Intestinal Tissue (Caecum) | Intestinal Fluid | Intestinal Tissue (Caecum) | Intestinal Fluid | Intestinal Tissue (Caecum) | Intestinal Fluid | Intestinal Tissue (Caecum) | |
| TAC | 6.21 ± 0.10 | 5.63 ± 0.38 | 5.26 ± 0.05 | 6.11 ± 0.07 | 6.42 ± 0.06 b | 6.42 ± 0.46 | 6.81 ± 0.31 a,b | 5.93 ± 0.55 |
| Enterococci | 4.71 ± 0.21 | 3.53 ± 0.49 | 4.48 ± 0.38 | 3.52 ± 0.24 | 6.25 ± 0.19 a,b | 3.98 ± 0.84 | 5.13 ± 0.44 c | 4.28 ± 0.48 |
| Coliforms | 3.54 ± 0.43 | 2.18 ± 0.21 | 3.72 ± 0.15 | 2.72 ± 0.36 | 5.20 ± 0.13 a,b | 4.32 ± 0.68 | 5.23 ± 0.36 a,b | 3.75 ± 0.89 |
| Enterobacteriaceae | 3.35 ± 0.31 | 2.21 ± 0.24 | 3.79 ± 0.24 | 2.63 ± 0.34 | 5.38 ± 0.20 a,b | 5.01 ± 0.70 | 5.77 ± 0.30 a,b | 4.80 ± 0.34 |
| Staphylococci | 3.58 ± 0.23 | 3.11 ± 0.24 | 4.75 ± 0.14 | 3.13 ± 0.17 | 5.46 ± 0.10 a,b | 4.45 ± 0.07 | 5.20 ± 0.31 a, | 3.95 ± 0.95 |
| E. coli | 3.34 ± 0.11 | 2.15 ± 0.25 | 3.74 ± 0.16 | 2.49 ± 0.18 | 5.34 ± 0.19 a,b | 4.55 ± 0.80 | 4.65 ± 0.72 a | 3.69 ± 1.10 |
| Clostridia | 5.94 ± 0.20 | 4.10 ± 0.44 | 6.00 ± 0.16 | 4.96 ± 0.31 | 7.36 ± 0.10 | 5.35 ± 0.40 | 5.89 ± 0.58 c | 5.88 ± 0.49 |
| Bifidobacteria | 5.94 ± 0.17 | 5.21 ± 0.54 | 6.97 ± 0.08 | 4.75 ± 0.22 | 6.74 ± 0.03 | 5.84 ± 0.66 | 7.02 ± 0.74 | 6.29 ± 0.90 |
| Lactobacilli | 4.58 ± 0.42 | 5.15 ± 0.65 | 6.85 ± 0.11 a | 5.46 ± 0.38 | 4.09 ± 0.04 b | 5.45 ± 0.15 | 6.70 ± 0.91 c | 5.58 ± 0.32 |
| Phylum | Relative Abundance (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| CD | CCD | DCD | DCCD | |||||
| Baseline | End | Baseline | End | Baseline | End | Baseline | End | |
| Firmicutes | 71.52 ± 3.59 | 69.69 ± 6.19 | 68.08 ± 2.61 | 72.60 ± 6.14 | 72.86 ± 1.71 | 75.99 ± 6.13 | 73.20 ± 6.80 | 71.14 ± 3.53 |
| Bacteroidetes | 22.63 ± 4.31 | 27.58 ± 2.69 | 20.89 ± 2.21 | 19.09 ± 2.33 | 25.16 ± 0.72 | 21.56 ± 4.38 | 20.07 ± 1.82 | 24.54 ± 8.22 |
| Actinobacteria | 0.03 ± 0.01 | 0.05 ± 0.02 | 0.07 ± 0.04 | 0.10 ± 0.06 | 1.82 ± 2.53 | 2.29 ± 1.86 | 0.13 ± 0.03 | 1.90 ± 2.10 |
| Verrucomicrobia | 0.13 ± 0.11 | 0.14 ± 0.06 | 9.69 ± 0.23 | 5.69 ± 4.96 | 0.11 ± 0.04 | 0.08 ± 0.01 | 6.35 ± 5.85 | 2.47 ± 2.29 |
| Tenericutes | 0.09 ± 0.01 | 0.18 ± 0.13 | 1.27 ± 0.12 | 2.52 ± 0.57 # | 0.05 ± 0.04 | 0.09 ± 0.12 | 0.25 ± 0.19 | 0.13 ± 0.11 |
| CD | CCD | DCD | DCCD | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | End | Baseline | End | Baseline | End | Baseline | End | |
| Lactic acid | 1.70 ±0.52 | 1.16 ± 0.02 | 1.79 ± 0.52 | 1.66 ± 0.53 | 1.94 ± 0.57 | 1.75 ± 0.52 | 2.01 ± 0.53 | 1.52 ± 0.22 |
| Acetic acid | 19.55 ± 5.56 | 24.25 ± 5.45 | 17.65 ± 5.10 | 30.48 ± 4.49 # | 34.81 ± 7.03 a,b | 24.49 ± 9.09 | 34.78 ± 8.10 a,b | 56.08 ± 9.86 a,b,# |
| Propionic acid | 1.93 ± 0.92 | 1.67 ± 0.46 | 2.00 ± 0.99 | 1.80 ± 0.19 | 1.89 ± 0.43 | 2.57 ± 0.86 | 1.78 ± 0.30 | 2.41 ± 0.75 |
| Isobutyric acid | 0.09 ± 0.05 | 0.29 ± 0.05 # | 0.10 ± 0.04 | 0.31 ± 0.14 # | 0.07 ± 0.02 | 0.20 ± 0.10 # | 0.07 ± 0.03 | 0.32± 0.06 # |
| Butyric acid | 1.41 ± 0.86 | 1.33 ± 0.68 | 1.28 ± 0.79 | 1.94 ± 0.39 | 1.37 ± 0.55 | 1.53 ± 0.71 | 1.43 ± 0.57 | 1.48 ± 0.91 |
| Isovaleric acid | 0.06 ± 0.01 | 0.12 ± 0.04 | 0.06 ± 0.01 | 0.11 ± 0.02 | 0.04 ± 0.02 | 0.10 ± 0.06 | 0.04 ± 0.02 | 0.10 ± 0.06 |
| Valeric acid | 0.11 ± 0.02 | 0.15 ± 0.03 | 0.10 ± 0.02 | 0.16 ± 0.04 | 0.10 ± 0.02 | 0.17 ± 0.07 | 0.10 ± 0.02 | 0.19 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kompoura, V.; Prapa, I.; Vasilakopoulou, P.B.; Mitropoulou, G.; Nelios, G.; Balafas, E.; Kostomitsopoulos, N.; Chiou, A.; Karathanos, V.T.; Bezirtzoglou, E.; et al. Corinthian Currants Supplementation Restores Serum Polar Phenolic Compounds, Reduces IL-1beta, and Exerts Beneficial Effects on Gut Microbiota in the Streptozotocin-Induced Type-1 Diabetic Rat. Metabolites 2023, 13, 415. https://doi.org/10.3390/metabo13030415
Kompoura V, Prapa I, Vasilakopoulou PB, Mitropoulou G, Nelios G, Balafas E, Kostomitsopoulos N, Chiou A, Karathanos VT, Bezirtzoglou E, et al. Corinthian Currants Supplementation Restores Serum Polar Phenolic Compounds, Reduces IL-1beta, and Exerts Beneficial Effects on Gut Microbiota in the Streptozotocin-Induced Type-1 Diabetic Rat. Metabolites. 2023; 13(3):415. https://doi.org/10.3390/metabo13030415
Chicago/Turabian StyleKompoura, Vasiliki, Ioanna Prapa, Paraskevi B. Vasilakopoulou, Gregoria Mitropoulou, Grigorios Nelios, Evangelos Balafas, Nikolaos Kostomitsopoulos, Antonia Chiou, Vaios T. Karathanos, Eugenia Bezirtzoglou, and et al. 2023. "Corinthian Currants Supplementation Restores Serum Polar Phenolic Compounds, Reduces IL-1beta, and Exerts Beneficial Effects on Gut Microbiota in the Streptozotocin-Induced Type-1 Diabetic Rat" Metabolites 13, no. 3: 415. https://doi.org/10.3390/metabo13030415
APA StyleKompoura, V., Prapa, I., Vasilakopoulou, P. B., Mitropoulou, G., Nelios, G., Balafas, E., Kostomitsopoulos, N., Chiou, A., Karathanos, V. T., Bezirtzoglou, E., Kourkoutas, Y., & Yanni, A. E. (2023). Corinthian Currants Supplementation Restores Serum Polar Phenolic Compounds, Reduces IL-1beta, and Exerts Beneficial Effects on Gut Microbiota in the Streptozotocin-Induced Type-1 Diabetic Rat. Metabolites, 13(3), 415. https://doi.org/10.3390/metabo13030415