The Potency of Goat Milk in Reducing the Induced Neurotoxic Effects of Valproic Acid in Rat Pups as a Rodent Model of Autism Spectrum Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Milk Products
2.2. Analysis of Fatty Acids in Cow and Goat Milk Products
2.3. Quantification of Milk Casein Content
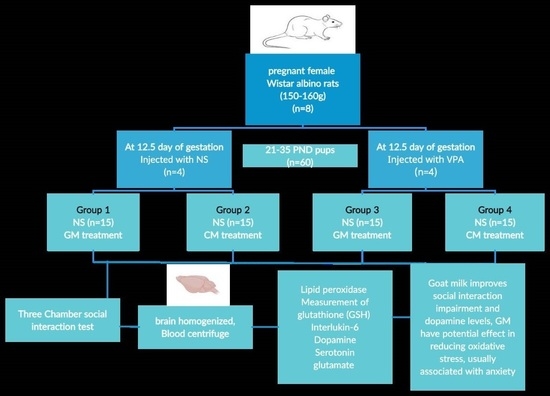
2.4. Experimental Design
2.5. Three-Chamber Sociability Test (TCSA)
2.6. Biochemical Assays in Brain Tissue and Serum
2.7. Statistical Analysis
3. Results
3.1. Milk Chemical Analysis
3.2. Analysis of Social Behavior
3.3. Analysis of Biochemical Variables
4. Discussion
5. Conclusions
6. Limitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicolini, C.; Fahnestock, M. The valproic acid-induced rodent model of autism. Expermental Neurol. 2018, 299, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Sébastien, C.; Morceau, F.; Dicato, M.; Diederich, M. Molecular and therapeutic potential and toxicity of valproic acid. J. Biomed. Biotechnol. 2010, 2010, 479364. [Google Scholar]
- O’Mahony, S.; Hyland, N.; Dinan, T.; Cryan, J. Maternal separation as a model of brain-gut axis dysfunction. Psychopharmacology. Psychopharmacology 2011, 214, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Butts, C.A.; Paturi, G.; Hedderley, D.I.; Martell, S.; Dinnan, H.; Stoklosinski, H.; Carpenter, E.A. Goat and cow milk differ in altering microbiota composition and fermentation products in rats with gut dysbiosis induced by amoxicillin. Food Funct. 2021, 12, 3104–3119. [Google Scholar] [CrossRef]
- Donovan, S.; Comstock, S. Human milk oligosaccharides influence neonatal mucosal and systemic immunity. Ann. Nutr. Metab. 2016, 69, 41–51. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Kling, D.; Leone, S.; Lawlor, N.; Huang, Y.; Feinberg, S.; Hill, D. The human milk oligosaccharide 20-fucosyllactose modulates cd14 expression in human enterocytes, thereby attenuating lps-induced inflammation. Gut 2016, 65, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Benton, D. The influence of children’s diet on their cognition and behavior. Eur. J. Nutr. 2008, 47, 25–37. [Google Scholar] [CrossRef]
- Tarr, A.J.; Galley, J.D.; Fisher, S.E.; Chichlowski, M.; Berg, B.M.; Bailey, M.T. The prebiotics 30 sialyllactose and 6 0 sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: Evidence for effects on the gut-brain axis. Brain Behav. Immun. 2015, 50, 166–177. [Google Scholar] [CrossRef] [Green Version]
- Whiteley, P.; Shattock, P. Biochemical aspects in autism spectrum disorders: Updating the opioid-excess theory and presenting new opportunities for biomedical intervention. Expert Opin. Ther. Targets 2002, 6, 175–183. [Google Scholar] [CrossRef]
- Nakano, T.; Sugawara, M.; Kawakami, H. Sialic acid in human milk: Composition and functions. Acta Paediatr. Taiwanica 2001, 42, 11–17. [Google Scholar]
- Jost, T.; Lacroix, C.; Braegger, C.; Chassard, C. Impact of human milk bacteria and oligosaccharides on neonatal gut microbiota establishment and gut health. Nutr. Rev. 2015, 73, 426–437. [Google Scholar] [CrossRef]
- Bienenstock, J.; Buck, R.H.; Linke, H.; Forsythe, P.; Stanisz, A.M.; Kunze, W.A. Fucosylated but not sialylated milk oligosaccharides diminish colon motor contractions. PLoS ONE 2013, 8, e76236. [Google Scholar] [CrossRef] [Green Version]
- Espejo-Carpio, F.J.; García-Moreno, P.J.; Pérez-Gálvez, R.; Morales-Medina, R.; Guadix, A.; Guadix, E.M. Effect of digestive enzymes on the bioactive properties of goat milk protein hydrolysates. Int. Dairy J. 2016, 54, 21–28. [Google Scholar] [CrossRef]
- Tomotake, H.; Okuyama, R.; Katagiri, M.; Fuzita, M.; Yamato, M.; Ota, F. Comparison between Holstein cow’s milk and Japanese-Saanen goat’s milk in fatty acid composition, lipid digestibility and protein profile. Biosci. Biotechnol. Biochem. 2006, 70, 2771–2774. [Google Scholar] [CrossRef]
- Teng, F.; Wang, P.; Yang, L.; Ma, Y.; Day, L. Quantification of fatty acids in human, cow, buffalo, goat, yak, and camel milk using an improved one-step GC-FID method. Food Anal. Methods 2017, 10, 2881–2891. [Google Scholar] [CrossRef]
- Ulaş, A.S.; Çakır, A.; Erbaş, O. Gluten and Casein: Their Roles in Psychiatric Disorders. J. Exp. Basic Med. Sci. 2022, 3, 13–21. [Google Scholar]
- Baspinar, B.; Yardimci, H. Gluten-Free Casein-Free Diet for Autism Spectrum Disorders: Can It Be Effective in Solving Behavioural and Gastrointestinal Problems? Eurasian J. Med. 2020, 52, 292. [Google Scholar] [CrossRef]
- Kumaresan, A.; Selma, C.; Reshma, N.V.; Jacinth, N.A. Quantitative analysis of casein precipitation from the various milk samples. J. Chem. Pharm. Res. 2017, 9, 113–115. [Google Scholar]
- Al-Askar, M.; Bhat, R.S.; Selim, M.; Al-Ayadhi, L.; El-Ansary, A. Postnatal treatment using curcumin supplements to amend the damage in VPA-induced rodent models of autism. BMC Complement. Altern. Med. 2017, 17, 259. [Google Scholar] [CrossRef] [Green Version]
- Hamzawy, M.A.; El-Ghandour, Y.B.; Abdel-Aziem, S.H.; Ali, Z.H. Leptin and camel milk abate oxidative stress status, genotoxicity induced in valproic acid rat model of autism. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418785514. [Google Scholar] [CrossRef] [Green Version]
- Moy, S.S.; Nadler, J.J.; Perez, A.; Barbaro, R.P.; Johns, J.M.; Magnuson, T.R.; Crawley, J.N. Sociability and preference for social novelty in five inbred strains: An approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004, 3, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Buzanovskii, V.A. Determination of proteins in blood. Part 1: Determination of total protein and albumin. Rev. J. Chem. 2017, 7, 79–124. [Google Scholar] [CrossRef]
- Hodgkinson, A.J.; Wallace, O.A.; Boggs, I.; Broadhurst, M.; Prosser, C.G. Gastric digestion of cow and goat milk: Impact of infant and young child in vitro digestion conditions. Food Chem. 2018, 245, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, L.; Zhou, R.; Zhang, J.; Xie, K.; Hou, Y.; Zhou, P. Cow’s milk αS1-casein is more sensitizing than goat’s milk αS1-casein in a mouse model. Food Funct. 2022, 13, 6484–6497. [Google Scholar] [CrossRef]
- Haenlein, G.F.W. Goat milk in human nutrition. Small Rumin. Res. 2004, 51, 155–163. [Google Scholar] [CrossRef]
- Idamokoro, E.M.; Muchenje, V.; Afolayan, A.J.; Hugo, A. Comparative fatty-acid profile and atherogenicity index of milk from free grazing Nguni, Boer and non-descript goats in South Africa. Pastoralism 2019, 9, 4. [Google Scholar] [CrossRef] [Green Version]
- Alfawaz, H.; Al-Onazi, M.; Bukhari, S.I.; Binobead, M.; Othman, N.; Algahtani, N.; El-Ansary, A. The Independent and Combined Effects of Omega-3 and Vitamin B12 in Ameliorating Propionic Acid Induced Biochemical Features in Juvenile Rats as Rodent Model of Autism. J. Mol. Neurosci. 2018, 66, 403–413. [Google Scholar] [CrossRef]
- Tartaglione, A.M.; Schiavi, S.; Calamandrei, G.; Trezza, V. Prenatal valproate in rodents as a tool to understand the neural underpinnings of social dysfunctions in autism spectrum disorder. Neuropharmacology 2019, 159, 107477. [Google Scholar] [CrossRef]
- Sengupta, P. The laboratory rat: Relating its age with human’s. Int. J. Prev. Med. 2013, 4, 624. [Google Scholar]
- Chaliha, D.; Albrecht, M.; Vaccarezza, M.; Takechi, R.; Lam, V.; Al-Salami, H.; Mamo, J. A systematic review of the valproic-acid-induced rodent model of autism. Dev. Neurosci. 2020, 42, 12–48. [Google Scholar] [CrossRef]
- Mony, T.; Lee, J.W.; Dreyfus, C.; DiCicco-Bloom, E.; Lee, H.J. Valproic acid exposure during early postnatal gliogenesis leads to autistic-like behaviors in rats. Clin. Psychopharmacol. Neurosci. 2016, 14, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunarti, S.; Nurliyani, N.; Tyas, A.S.A.; Kristian, S.D.; Prasetyastuti, P. The influence of goat milk and soybean milk kefir on IL-6 and crp levels in diabetic rats. Rom. J. Diabetes Nutr. Metab. Dis. 2015, 22, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Essa, M.M.; Braidy, N.; Vijayan, K.R.; Subash, S.; Guillemin, G.J. Excitotoxicity in the pathogenesis of autism. Neurotox. Res. 2013, 23, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Kosillo, P.; Bateup, H. Dopaminergic Dysregulation in Syndromic Autism Spectrum Disorders: Insights From Genetic Mouse Models. Front. Neural Circuits 2021, 15, 700968. [Google Scholar] [CrossRef] [PubMed]
- Froemke, R.C.; Young, L.J. Oxytocin, neural plasticity, and social behavior. Annu. Rev. Neurosci. 2021, 44, 359–381. [Google Scholar] [CrossRef]
- Garbarino, V.R.; Gilman, T.L.; Daws, L.C.; Gould, G.G. Extreme enhancement or depletion of serotonin transporter function and serotonin availability in autism spectrum disorder. Pharmacol. Res. 2019, 140, 85–99. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Deng, J.; Lin, J.; Hu, C.; Li, Q.; Xu, X. Social Deficits and Repetitive Behaviors Are Improved by Early Postnatal Low-Dose VPA Intervention in a Novel shank3-Deficient Zebrafish Model. Front. Neurosci. 2021, 15, 1125. [Google Scholar] [CrossRef]
- Alghamdi, M.; Al-Ayadhi, L.; Hassan, W.M.; Bhat, R.S.; Alonazi, M.A.; El-Ansary, A. Bee Pollen and Probiotics May Alter Brain Neuropeptide Levels in a Rodent Model of Autism Spectrum Disorders. Metabolites 2022, 12, 562. [Google Scholar] [CrossRef]
- El-Ansary, A.; Chirumbolo, S.; Bhat, R.S.; Dadar, M.; Ibrahim, E.M.; Bjørklund, G. The role of lipidomics in autism spectrum disorder. Mol. Diagn. Ther. 2020, 24, 31–48. [Google Scholar] [CrossRef]
- Moreno-Fernández, J.; López-Aliaga, I.; García-Burgos, M.; Alférez, J.M.; Díaz-Castro, J. Fermented goat milk consumption enhances brain molecular functions during Iron deficiency anemia recovery. Nutrients 2019, 11, 2394. [Google Scholar] [CrossRef] [Green Version]
- Bourre, J.M. Dietary omega-3 fatty acids and psychiatry: Mood, behaviour, stress, depression, dementia and aging. J. Nutr. Health Aging 2005, 9, 31–38. [Google Scholar]
- McNamara, I.; Borella, A.W.; Bialowas, L.A.; Whitaker-Azmitia, P.M. Further studies in the developmental hyperserotonemia model (DHS) of autism: Social, behavioral and peptide changes. Brain Res. 2008, 1189, 203–214. [Google Scholar] [CrossRef]
- Miyazaki, K.; Narita, N.; Narita, M. Maternal administration of thalidomide or valproic acid causes abnormal serotonergic neurons in the offspring: Implication for pathogenesis of autism. Int. J. Dev. Neurosci. 2005, 23, 287–297. [Google Scholar] [CrossRef]
- Zieminska, E.; Toczylowska, B.; Diamandakis, D.; Hilgier, W.; Filipkowski, R.; Polowy, R.; Lazarewicz, J.W. Glutamate, glutamine and GABA levels in rat brain measured using MRS, HPLC and NMR methods in study of two models of autism. Front. Mol. Neurosci. 2018, 11, 418. [Google Scholar] [CrossRef] [Green Version]
- Bjørklund, G.; Dos, M.D.; Maes, M.; Dadar, M.; Frye, R.E.; Peana, M.; Chirumbolo, S. The impact of glutathione metabolism in autism spectrum disorder. Pharmacol. Res. 2021, 166, 105437. [Google Scholar] [CrossRef]
- El-Ansary, A.; Al-Ayadhi, L. GABAergic/glutamatergic imbalance relative to excessive neuroinflammation in autism spectrum disorders. J. Neuroinflamm. 2014, 11, 189. [Google Scholar] [CrossRef] [Green Version]
- Ruhela, R.K.; Soni, S.; Sarma, P.; Prakash, A.; Medhi, B. Negative geotaxis: An early age behavioral hallmark to VPA rat model of autism. Ann. Neurosci. 2019, 26, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Navarro, F.; Pearson, D.A.; Fatheree, N.; Mansour, R.; Hashmi, S.S.; Rhoads, J.M. Are ‘leaky gut’and behavior associated with gluten and dairy containing diet in children with autism spectrum disorders? Nutr. Neurosci. 2015, 18, 177–185. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The Gut Microbiota-Brain axis in Behaviour and Brain Disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Srikantha, P.; Mohajeri, M.H. The possible role of the microbiota-gut-brain-axis in autism spectrum disorder. Int. J. Mol. Sci. 2019, 20, 2115. [Google Scholar] [CrossRef] [Green Version]
- Al-Ayadhi, L.; Elamin, N.E. Camel milk as a potential therapy as an antioxidant in autism spectrum disorder (ASD). Evid.-Based Complement. Altern. Med. 2013, 2013, 602834. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Xu, Y.; Zhang, X.; Duan, G.; Song, C.; Zhu, C. Gut microbiota changes in patients with autism spectrum disorders. J. Psychiatr. Res. 2020, 129, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Abuaish, S.; Al-Otaibi, N.; Abujamel, T.; Alzahrani, S.; Alotaibi, S.; AlShawakir, Y.; Aabed, K.; El-Ansary, A. Fecal Transplant and Bifidobacterium Treatments Modulate Gut Clostridium Bacteria and Rescue Social Impairment and Hippocampal BDNF Expression in a Rodent Model of Autism. Brain Sci. 2021, 11, 1038. [Google Scholar] [CrossRef] [PubMed]
- Needham, B.; Funabashi, M.; Adame, M.; Wang, Z.; Boktor, J.; Haney, J. A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature 2022, 607, 647–653. [Google Scholar] [CrossRef]
- Chau, K.F. The Effect of Sulforaphane on Valproic Acid on Mice Model of Autism. Ph.D. Thesis, Chinese University of Hong Kong, Hong Kong, China, 2017. [Google Scholar]






| Fatty Acid Profile | Goat’s Milk | Cow’s Milk | p-Value |
|---|---|---|---|
| Saturated Fatty Acid | 2.80 g/100 g | 1.96 g/100 g | 0.8288 |
| Unsaturated Fatty Acid | 1.24 g/100 g | 1.03 g/100 g | 0.9083 |
| Monounsaturated Fatty Acid | 0.91 g/100 g | 0.82 g/100 g | 0.9481 |
| Polyunsaturated Fatty Acid | 0.19 g/100 g | 0.14 g/100 g | 0.8518 |
| Trans Fatty Acid | 0.14 g/100 g | 0.07 g/100 g | 0.6985 |
| C18:2n6c (Linoleic) | 3.81% | 3.69% | 0.984 |
| C18:3n3 (A-Linolenic) | 0.57% | 0.49% | 0.925 |
| Ratio of ω-3 and ω-6 precursors | 1.27:0.19 | 0.163333:1.23 |
| Name | Temperature | PH | ML | Casein g | Casein% | p-Value |
|---|---|---|---|---|---|---|
| Goat’s milk | 50 °C | 4.6 | 60 | 1.95 g | 3.2% | 0.8850 |
| Cow’s milk | 50 °C | 4.6 | 60 | 2.43 g | 4.0% |
| Groups | Initial Weight (g) | Final Weight (g) | Body Weight Gain (g) |
|---|---|---|---|
| Control Cow | 35.67 ± 1.116 | 84.47 ± 3.022 | 48.80 ± 2.698 |
| VPA Cow | 37.27 ± 1.080 * | 94.60 ± 4.022 | 57.33 ± 3.915 |
| Control Goat | 36.07 ± 0.9333 * | 67.27 ± 3.087 *** | 31.20 ± 2.675 *** |
| VPA Goat | 41.07 ± 1.672 * | 95.53 ± 3.290 *** | 54.47 ± 2.635 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-dossari, A.M.; Al-Harbi, L.N.; Al-Otaibi, N.M.; Almubarak, A.; Almnaizel, A.T.; Alshammari, G.M.; Shamlan, G.; Alshatwi, A.A.; El-Ansary, A. The Potency of Goat Milk in Reducing the Induced Neurotoxic Effects of Valproic Acid in Rat Pups as a Rodent Model of Autism Spectrum Disorder. Metabolites 2023, 13, 497. https://doi.org/10.3390/metabo13040497
Al-dossari AM, Al-Harbi LN, Al-Otaibi NM, Almubarak A, Almnaizel AT, Alshammari GM, Shamlan G, Alshatwi AA, El-Ansary A. The Potency of Goat Milk in Reducing the Induced Neurotoxic Effects of Valproic Acid in Rat Pups as a Rodent Model of Autism Spectrum Disorder. Metabolites. 2023; 13(4):497. https://doi.org/10.3390/metabo13040497
Chicago/Turabian StyleAl-dossari, Alhanouf Mohammed, Laila Naif Al-Harbi, Norah M. Al-Otaibi, Abdullah Almubarak, Ahmed Tayseer Almnaizel, Ghedeir M. Alshammari, Ghalia Shamlan, Ali A. Alshatwi, and Afaf El-Ansary. 2023. "The Potency of Goat Milk in Reducing the Induced Neurotoxic Effects of Valproic Acid in Rat Pups as a Rodent Model of Autism Spectrum Disorder" Metabolites 13, no. 4: 497. https://doi.org/10.3390/metabo13040497
APA StyleAl-dossari, A. M., Al-Harbi, L. N., Al-Otaibi, N. M., Almubarak, A., Almnaizel, A. T., Alshammari, G. M., Shamlan, G., Alshatwi, A. A., & El-Ansary, A. (2023). The Potency of Goat Milk in Reducing the Induced Neurotoxic Effects of Valproic Acid in Rat Pups as a Rodent Model of Autism Spectrum Disorder. Metabolites, 13(4), 497. https://doi.org/10.3390/metabo13040497