Urine Metabolomic Signature of People Diagnosed with Balkan Endemic Nephropathy and Other Types of Chronic Kidney Disease Compared with Healthy Subjects in Romania
Abstract
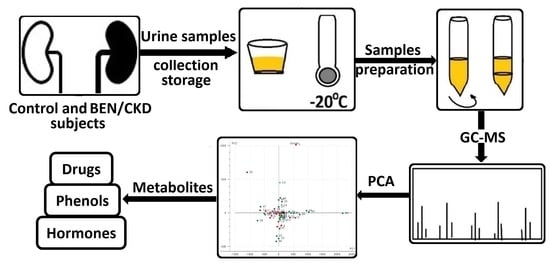
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solvents
2.2. Demographic Characteristics
2.3. Urine Sample Collection
- (1)
- Healthy control volunteers (n = 35; code names 23, 25, 28–36, 38–61) who are family members of BEN patients (n = 8) or live in BEN areas (“endemic controls”) (n = 27); these were self-selected by availability. We ensured that only people without any documented history of kidney disease and not currently taking prescribed medication participated in our study. Urine samples (0.66 male/female ratio) collected during the first part of the day served as controls for the samples collected from the BEN patients.
- (2)
- Patients with nephrological diseases other than BEN (n = 5; code names C1, C2, C3, C4, and C5) registered at the Clinical County Hospital, Timisoara, Romania, served as disease controls (1.5 male/female ratio).
- (3)
- Patients diagnosed with BEN (n = 25; code names 1–22, 24, 26, and 27) under hemodialysis at Renamed Centre, Drobeta-Turnu Severin, Mehedinti County. Patients (0.71 male/female ratio) were residents of Erghevita, Bistrita and Valea Izvorului villages in Mehedinti County. The subjects were self-selected during visits for hemodialysis sessions performed at the clinic, and none was suspected to have urinary tract tumors.
2.4. Urine Sample Extraction
2.5. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.6. Principal Component Analysis (PCA) and Classical Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin-Cleary, C.; Ortiz, A. CKD Hotspots around the World: Where, Why and What the Lessons Are. A CKJ Review Series. Clin. Kidney J. 2014, 7, 519–523. [Google Scholar] [CrossRef]
- Stefanovic, V. Balkan Endemic Nephropathy. A Reaprrisal after Forty Years. FACTA Univ. 1999, 6, 53–58. [Google Scholar]
- Baudoux, T.; Jadot, I.; Declèves, A.-E.; Antoine, M.-H.; Colet, J.-M.; Botton, O.; De Prez, E.; Pozdzik, A.; Husson, C.; Caron, N.; et al. Experimental Aristolochic Acid Nephropathy: A Relevant Model to Study AKI-to-CKD Transition. Front. Med. 2022, 9, 2870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Luo, P.; Chen, J.; Yang, C.; Xia, F.; Zhang, J.; Tang, H.; Liu, D.; Gu, L.; Shi, Q.; et al. Dissection of Targeting Molecular Mechanisms of Aristolochic Acid-Induced Nephrotoxicity via a Combined Deconvolution Strategy of Chemoproteomics and Metabolomics. Int. J. Biol. Sci. 2022, 18, 2003–2017. [Google Scholar] [CrossRef] [PubMed]
- Hocher, B.; Adamski, J. Metabolomics for Clinical Use and Research in Chronic Kidney Disease. Nat. Rev. Nephrol. 2017, 13, 269–284. [Google Scholar] [CrossRef]
- Gifford, F.J.; Gifford, R.M.; Eddleston, M.; Dhaun, N. Endemic Nephropathy Around the World. Kidney Int. Rep. 2017, 2, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, S.; Nafar, M. An Update of Urine and Blood Metabolomics in Chronic Kidney Disease. Biomark. Med. 2019, 13, 577–596. [Google Scholar] [CrossRef]
- Bouatra, S.; Aziat, F.; Mandal, R.; Guo, A.C.; Wilson, M.R.; Knox, C.; Bjorndahl, T.C.; Krishnamurthy, R.; Saleem, F.; Liu, P.; et al. The Human Urine Metabolome. PLoS ONE 2013, 8, e73076. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, M.; Pourfarzam, M. A Review of Strategies for Untargeted Urinary Metabolomic Analysis Using Gas Chromatography–Mass Spectrometry. Metabolomics 2020, 16, 66. [Google Scholar] [CrossRef]
- Abbiss, H.; Maker, G.; Trengove, R. Metabolomics Approaches for the Diagnosis and Understanding of Kidney Diseases. Metabolites 2019, 9, 34. [Google Scholar] [CrossRef]
- Jain, A.; Li, X.H.; Chen, W.N. An Untargeted Fecal and Urine Metabolomics Analysis of the Interplay between the Gut Microbiome, Diet and Human Metabolism in Indian and Chinese Adults. Sci. Rep. 2019, 9, 9191. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, W.; Li, R.; Wang, M.; Chen, C.; Zeng, R.; Deng, Y. Systematic Variations Associated with Renal Disease Uncovered by Parallel Metabolomics of Urine and Serum. BMC Syst. Biol. 2012, 6, S14. [Google Scholar] [CrossRef]
- Gromski, P.S.; Muhamadali, H.; Ellis, D.I.; Xu, Y.; Correa, E.; Turner, M.L.; Goodacre, R. A Tutorial Review: Metabolomics and Partial Least Squares-Discriminant Analysis—A Marriage of Convenience or a Shotgun Wedding. Anal. Chim. Acta 2015, 879, 10–23. [Google Scholar] [CrossRef]
- Grissa, D.; Pétéra, M.; Brandolini, M.; Napoli, A.; Comte, B.; Pujos-Guillot, E. Feature Selection Methods for Early Predictive Biomarker Discovery Using Untargeted Metabolomic Data. Front. Mol. Biosci. 2016, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Gromski, P.; Xu, Y.; Kotze, H.; Correa, E.; Ellis, D.; Armitage, E.; Turner, M.; Goodacre, R. Influence of Missing Values Substitutes on Multivariate Analysis of Metabolomics Data. Metabolites 2014, 4, 433–452. [Google Scholar] [CrossRef]
- Mantle, P.; Modalca, M.; Nicholls, A.; Tatu, C.; Tatu, D.; Toncheva, D. Comparative 1H NMR Metabolomic Urinalysis of People Diagnosed with Balkan Endemic Nephropathy, and Healthy Subjects, in Romania and Bulgaria: A Pilot Study. Toxins 2011, 3, 815–833. [Google Scholar] [CrossRef] [PubMed]
- Trnacevic, S.; Nislic, E.; Trnacevic, E.; Tulumovic, E. Early Screening of Balkan Endemic Nephropathy. Mater. Socio Medica 2017, 29, 207. [Google Scholar] [CrossRef]
- Korenius, T.; Laurikkala, J.; Juhola, M. On Principal Component Analysis, Cosine and Euclidean Measures in Information Retrieval. Inf. Sci. 2007, 177, 4893–4905. [Google Scholar] [CrossRef]
- Ringnér, M. What Is Principal Component Analysis? Nat. Biotechnol. 2008, 26, 303–304. [Google Scholar] [CrossRef]
- Waikar, S.S.; Sabbisetti, V.S.; Bonventre, J.V. Normalization of Urinary Biomarkers to Creatinine during Changes in Glomerular Filtration Rate. Kidney Int. 2010, 78, 486–494. [Google Scholar] [CrossRef]
- Khalid, T.; White, P.; De Lacy Costello, B.; Persad, R.; Ewen, R.; Johnson, E.; Probert, C.S.; Ratcliffe, N. A Pilot Study Combining a GC-Sensor Device with a Statistical Model for the Identification of Bladder Cancer from Urine Headspace. PLoS ONE 2013, 8, e69602. [Google Scholar] [CrossRef]
- Wang, M.; Xie, R.; Jia, X.; Liu, R. Urinary Volatile Organic Compounds as Potential Biomarkers in Idiopathic Membranous Nephropathy. Med. Princ. Pract. 2017, 26, 375–380. [Google Scholar] [CrossRef]
- Smith, S.; Burden, H.; Persad, R.; Whittington, K.; de Lacy Costello, B.; Ratcliffe, N.M.; Probert, C.S. A Comparative Study of the Analysis of Human Urine Headspace Using Gas Chromatography–Mass Spectrometry. J. Breath Res. 2008, 2, 037022. [Google Scholar] [CrossRef]
- Yu, H.; Wu, Z.; Wei, Z.; Zhai, Y.; Ru, S.; Zhao, Q.; Wang, J.; Han, S.; Wei, Y. N-Formylation of Amines Using Methanol as a Potential Formyl Carrier by a Reusable Chromium Catalyst. Commun. Chem. 2019, 2, 15. [Google Scholar] [CrossRef]
- Maki, T.; Takeda, K. Benzoic Acid and Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000; ISBN 978-3-527-30385-4. [Google Scholar]
- Fiege, H. Cresols and Xylenols. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; pp. 419–461. ISBN 978-3-527-30673-2. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) Scientific Opinion on the Re-Evaluation of Butylated Hydroxyanisole—BHA (E 320) as a Food Additive. EFSA J. 2011, 9, 2392. [CrossRef]
- Chiyanzu, I.; Hansell, E.; Gut, J.; Rosenthal, P.J.; McKerrow, J.H.; Chibale, K. Synthesis and Evaluation of Isatins and Thiosemicarbazone Derivatives against Cruzain, Falcipain-2 and Rhodesain. Bioorg. Med. Chem. Lett. 2003, 13, 3527–3530. [Google Scholar] [CrossRef]
- Heinig, M.; Johnson, R.J. Role of Uric Acid in Hypertension, Renal Disease, and Metabolic Syndrome. Cleve. Clin. J. Med. 2006, 73, 1059–1064. [Google Scholar] [CrossRef]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A. Normal and Pathologic Concentrations of Uremic Toxins. J. Am. Soc. Nephrol. JASN 2012, 23, 1258–1270. [Google Scholar] [CrossRef]
- Lim, Y.J.; Sidor, N.A.; Tonial, N.C.; Che, A.; Urquhart, B.L. Uremic Toxins in the Progression of Chronic Kidney Disease and Cardiovascular Disease: Mechanisms and Therapeutic Targets. Toxins 2021, 13, 142. [Google Scholar] [CrossRef]
- Lauriola, M.; Farré, R.; Evenepoel, P.; Overbeek, S.A.; Meijers, B. Food-Derived Uremic Toxins in Chronic Kidney Disease. Toxins 2023, 15, 116. [Google Scholar] [CrossRef]
- Ni, Y.; Su, M.; Qiu, Y.; Chen, M.; Liu, Y.; Zhao, A.; Jia, W. Metabolic Profiling Using Combined GC-MS and LC-MS Provides a Systems Understanding of Aristolochic Acid-Induced Nephrotoxicity in Rat. FEBS Lett. 2007, 581, 707–711. [Google Scholar] [CrossRef]
- Podea, R.; Culea, M.; Fromondi, L. The Determination of the Therapeutic Compounds from Aristolochia Clematitis by GC/MS. Stud. Univ. Babeş-Bolyai Phys. 2001, 378–384. [Google Scholar]
- Duan, L.; Guo, L.; Wang, L.; Yin, Q.; Zhang, C.-M.; Zheng, Y.-G.; Liu, E.-H. Application of Metabolomics in Toxicity Evaluation of Traditional Chinese Medicines. Chin. Med. 2018, 13, 60. [Google Scholar] [CrossRef]
- Hu, X.; Shen, J.; Pu, X.; Zheng, N.; Deng, Z.; Zhang, Z.; Li, H. Urinary Time- or Dose-Dependent Metabolic Biomarkers of Aristolochic Acid-Induced Nephrotoxicity in Rats. Toxicol. Sci. 2017, 156, kfw244. [Google Scholar] [CrossRef]
- Feder, G.L.; Tatu, C.A.; Orem, W.H.; Paunescu, V.; Dumitrascu, V.; Szilagyi, D.N.; Finkelman, R.B.; Margineanu, F.; Schneider, F. Weathered Coal Deposits and Balkan Endemic Nephropathy. FACTA Univ. 2002, 9, 34–38. [Google Scholar]
- Farzan, S.F.; Chen, Y.; Trachtman, H.; Trasande, L. Urinary Polycyclic Aromatic Hydrocarbons and Measures of Oxidative Stress, Inflammation and Renal Function in Adolescents: NHANES 2003–2008. Environ. Res. 2016, 144, 149–157. [Google Scholar] [CrossRef]

| Category | n (%) |
|---|---|
| Male | 28 (43.1%) |
| Female | 37 (56.9%) |
| Group categories of study population | |
| Healthy volunteers | 35 (53.9%) |
| BEN patients | 25 (38.5%) |
| CKD patients | 5 (7.7%) |
| Rural population | 60 (92.3%) |
| Urban population | 5 (7.7%) |
| Mean age ± standard deviation years | |
| Age (mean ± standard deviation) | 65.09 ± 2.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ordodi, V.L.; Hădărugă, N.G.; Hădărugă, D.I.; Lukinich-Gruia, A.T.; Mărgineanu, M.; Tatu, C.A.; Păunescu, V. Urine Metabolomic Signature of People Diagnosed with Balkan Endemic Nephropathy and Other Types of Chronic Kidney Disease Compared with Healthy Subjects in Romania. Metabolites 2023, 13, 609. https://doi.org/10.3390/metabo13050609
Ordodi VL, Hădărugă NG, Hădărugă DI, Lukinich-Gruia AT, Mărgineanu M, Tatu CA, Păunescu V. Urine Metabolomic Signature of People Diagnosed with Balkan Endemic Nephropathy and Other Types of Chronic Kidney Disease Compared with Healthy Subjects in Romania. Metabolites. 2023; 13(5):609. https://doi.org/10.3390/metabo13050609
Chicago/Turabian StyleOrdodi, Valentin L., Nicoleta G. Hădărugă, Daniel I. Hădărugă, Alexandra T. Lukinich-Gruia, Mihaela Mărgineanu, Călin A. Tatu, and Virgil Păunescu. 2023. "Urine Metabolomic Signature of People Diagnosed with Balkan Endemic Nephropathy and Other Types of Chronic Kidney Disease Compared with Healthy Subjects in Romania" Metabolites 13, no. 5: 609. https://doi.org/10.3390/metabo13050609
APA StyleOrdodi, V. L., Hădărugă, N. G., Hădărugă, D. I., Lukinich-Gruia, A. T., Mărgineanu, M., Tatu, C. A., & Păunescu, V. (2023). Urine Metabolomic Signature of People Diagnosed with Balkan Endemic Nephropathy and Other Types of Chronic Kidney Disease Compared with Healthy Subjects in Romania. Metabolites, 13(5), 609. https://doi.org/10.3390/metabo13050609