Chemical Characterization and In Vitro Gas Production Kinetics of Alternative Feed Resources for Small Ruminants in the Maltese Islands
Abstract
:1. Introduction
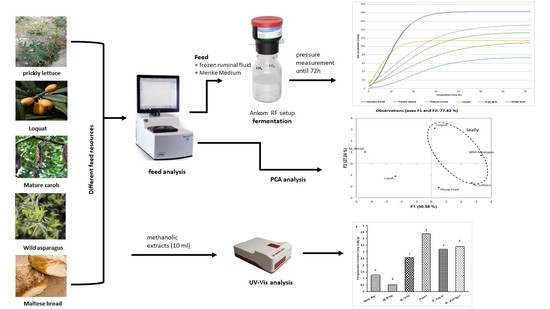
2. Materials and Methods
2.1. Feed Resources
Analysis
2.2. Proximate Analysis
2.3. Inoculum Sources
2.4. In Vitro Gas Production
2.5. Physicochemical and Antioxidant Activity of Substrates Extracts
2.6. UV Analysis and Absorbance
Color intensity = (A420 × DF) + (A520 × DF) + (A620 × DF)
Flavonoid Ratio = A420/A520
Anthocyanin content (mg/kg) = 1000 × Vs × DF × A520/ε
2.7. Folin–Ciocalteu Test for Polyphenols/Total Phenolic Content
2.8. Antioxidant Scavenging Assay
2.9. Statistics Analysis
3. Results
3.1. Proximate Analysis
3.2. In Vitro Gas Production and Fermentation Kinetics
3.3. UV-Vis Analysis
Antioxidant Activity Assessment
3.4. FC Test Results
3.5. DPPH Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations (UN). World Population Prospects: The 2017 Revision, Key Findings and Advance Tables; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Attard, E.; Pacioni, P. The Phytochemical and In Vitro Pharmacological Testing of Maltese Medicinal Plants. In Bioactive Compounds in Phytomedicine; InTech: Rijeka, Croatia, 2012; pp. 93–112. [Google Scholar]
- Estévez, M. Critical overview of the use of plant antioxidants in the meat industry: Opportunities, innovative applications and future perspectives. Meat Sci. 2021, 181, 108610. [Google Scholar] [CrossRef]
- Menke, K.H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Attard, E. A rapid microtitre plate Folin-Ciocalteu method for the assessment of polyphenols. Open Life Sci. 2013, 8, 48–53. [Google Scholar] [CrossRef]
- Prieto, J.M. Procedure: Preparation of DPPH Radical, and antioxidant scavenging assay. DPPH Microp. Protoc. 2012, 7–9. [Google Scholar]
- Bueno, I.C.S.; Cabral Filho, S.L.S.; Gobbo, S.P.; Louvandini, H.; Vitti, D.M.S.S.; Abdalla, A.L. Influence of inoculum source in a gas production method. Anim. Feed Sci. Technol. 2005, 123, 95–105. [Google Scholar] [CrossRef]
- Chitrakar, B.; Zhang, M.; Adhikari, B. Asparagus (Asparagus officinalis): Processing effect on nutritional and phytochemical composition of spear and hard-stem byproducts. Trends Food Sci. Technol. 2019, 93, 1–11. [Google Scholar] [CrossRef]
- Drinkwater, J.M.; Tsao, R.; Liu, R.; Defelice, C.; Wolyn, D.J. Effects of cooking on rutin and glutathione concentrations and antioxidant activity of green asparagus (Asparagus officinalis) spears. J. Funct. Foods 2015, 12, 342–353. [Google Scholar] [CrossRef]
- Gonnella, M.; Durante, M.; Caretto, S.; D’Imperio, M.; Renna, M. Quality assessment of ready-to-eat asparagus spears as affected by conventional and sous-vide cooking methods. LWT 2018, 92, 161–168. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Xu, C.; Li, X. Biological Activities of Extracts from Loquat (Eriobotrya japonica Lindl.): A Review. Int. J. Mol. Sci. 2016, 17, 1983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sergio, L.; Boari, F.; Di Venere, D.; Gonnella, M.; Cantore, V.; Renna, M. Quality Evaluation of Wild and Cultivated Asparagus: A Comparison between Raw and Steamed Spears. Agriculture 2021, 11, 1213. [Google Scholar] [CrossRef]
- Maeda, T.; Honda, K.; Sonoda, T.; Motoki, S.; Inoue, K.; Suzuki, T.; Oosawa, K.; Suzuki, M. Light Condition Influences Rutin and Polyphenol Contents in Asparagus Spears in the Mother-fern Culture System during the Summer–Autumn Harvest. J. Jpn. Soc. Hortic. Sci. 2010, 79, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Lebeda, A.; Křístková, E.; Kitner, M.; Mieslerová, B.; Jemelková, M.; Pink, D.A.C. Resistance of wild Lactuca genetic resources to diseases and pests, and their exploitation in lettuce breeding. Acta Hortic. 2015, 1101, 133–140. [Google Scholar] [CrossRef]
- Kim, S.W.; Huh, W.; Kim, S.Y.; Kim, J.S.; Lee, K.J. Isolation and Characterization of Bio-active Materials from Prickly Lettuce (Lactuca serriola). Sung-Il Moon. J. Life Sci. 2009, 19, 206–212. [Google Scholar]
- Costa, B.P.; Carpiné, D.; da Silva Bambirra Alves, F.E.; Barbi, R.C.T.; de Melo, A.M.; Ikeda, M.; Ribani, R.H. Thermal, structural, morphological and bioactive characterization of acid and neutral modified loquat (Eriobotrya japonica Lindl.) seed starch and its by-products. J. Therm. Anal. Calorim. 2022, 147, 6721–6737. [Google Scholar] [CrossRef]
- Dhiman, A.; Suhag, R.; Thakur, D.; Gupta, V.; Prabhakar, P.K. Current Status of Loquat (Eriobotrya Japonica Lindl.): Bioactive Functions, Preservation Approaches, and Processed Products. Food Rev. Int. 2022, 38, 286–316. [Google Scholar] [CrossRef]
- Turola Barbi, R.C.; Teixeira, G.L.; Hornung, P.S.; Ávila, S.; Hoffmann-Ribani, R. Eriobotrya japonica seed as a new source of starch: Assessment of phenolic compounds, antioxidant activity, thermal, rheological and morphological properties. Food Hydrocoll. 2018, 77, 646–658. [Google Scholar] [CrossRef]
- Hernández, F.; Madrid, J.; Cerón, J.J.; Pulgar, M.A.; Cid, J.M. Utilisation of lemon (Citrus limon) and loquat (Eribotrya japonica) tree leaves alone or with NH3-treated straw for goats. J. Sci. Food Agric. 1998, 77, 133–139. [Google Scholar] [CrossRef]
- Khouya, T.; Ramchoun, M.; Elbouny, H.; Hmidani, A.; Bouhlali, E.d.T.; Alem, C. Loquat (Eriobotrya japonica (Thunb) Lindl.): Evaluation of nutritional value, polyphenol composition, antidiabetic effect, and toxicity of leaf aqueous extract. J. Ethnopharmacol. 2022, 296, 115473. [Google Scholar] [CrossRef] [PubMed]
- Bakker, R.R.; Elbersen, H.W. Managing ash content and quality in herbaceous biomass: An analysis from plant to product managing ash content and-quality in herbaceous biomass: An analysis from plant to product. In Proceedings of the 14th European Biomass Conference, Paris, France, 17–21 October 2005; p. 21. [Google Scholar]
- Simsek, S.; Ozcan, M.M.; Al Juhaimi, F.; ElBabiker, E.; Ghafoor, K. Amino Acid and Sugar Contents of Wild and Cultivated Carob (Ceratonia siliqua) Pods Collected in Different Harvest Periods. Chem. Nat. Compd. 2017, 53, 1008–1009. [Google Scholar] [CrossRef]
- Richane, A.; Ismail, H.B.; Darej, C.; Attia, K.; Moujahed, N. Potential of Tunisian carob pulp as feed for ruminants: Chemical composition and in vitro assessment. Trop. Anim. Health Prod. 2022, 54, 58. [Google Scholar] [CrossRef]
- Kumazawa, S.; Taniguchi, M.; Suzuki, Y.; Shimura, M.; Kwon, M.S.; Nakayama, T. Antioxidant Activity of Polyphenols in Carob Pods. J. Agric. Food Chem. 2002, 50, 373–377. [Google Scholar] [CrossRef]
- Calislar, S.; Kaplan, Y. Effects of carob (Ceratonia siliqua) pod byproduct on quail performance, egg characteristics, fatty acids, and cholesterol levels. Rev. Bras. Zootec. 2017, 46, 113–117. [Google Scholar] [CrossRef] [Green Version]
- Youssef, M.K.E.; El-Manfaloty, M.M.; Ali, H.M. Assessment of proximate chemical composition, nutritional status, fatty acid composition and phenolic compounds of carob (Ceratonia siliqua L.). Food Public Health 2013, 3, 304–308. [Google Scholar]
- Kaltenegger, A.; Humer, E.; Stauder, A.; Zebeli, Q. Feeding of bakery by-products in the replacement of grains enhanced milk performance, modulated blood metabolic profile, and lowered the risk of rumen acidosis in dairy cows. J. Dairy Sci. 2020, 103, 10122–10135. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, L.; Luciano, A.; Ottoboni, M.; Manoni, M.; Ferrari, L.; Marchis, D.; Tretola, M. Recycling food leftovers in feed as opportunity to increase the sustainability of livestock production. J. Clean. Prod. 2021, 294, 126290. [Google Scholar] [CrossRef]
- Guiroy, P.J.; Fox, D.G.; Beermann, D.H.; Ketchen, D.J. Performance and meat quality of beef steers fed corn-based or bread by-product-based diets. J. Anim. Sci. 2000, 78, 784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humer, E.; Aditya, S.; Kaltenegger, A.; Klevenhusen, F.; Petri, R.M.; Zebeli, Q. Graded substitution of grains with bakery by-products modulates ruminal fermentation, nutrient degradation, and microbial community composition in vitro. J. Dairy Sci. 2018, 101, 3085–3098. [Google Scholar] [CrossRef] [Green Version]
- Chino Velasquez, L.B.; Molina-Botero, I.C.; Moscoso Muñoz, J.E.; Gómez Bravo, C. Relationship between Chemical Composition and In Vitro Methane Production of High Andean Grasses. Animals 2022, 12, 2348. [Google Scholar] [CrossRef] [PubMed]
- Váradyová, Z.; Baran, M.; Zeleňák, I. Comparison of two in vitro fermentation gas production methods using both rumen fluid and faecal inoculum from sheep. Anim. Feed. Sci. Technol. 2005, 123, 81–94. [Google Scholar] [CrossRef]
- Aderinboye, R.Y.; Akinlolu, A.O.; Adeleke, M.A.; Najeem, G.O.; Ojo, V.O.A.; Isah, O.A.; Babayemi, O.J. In vitro gas production and dry matter degradation of four browse leaves using cattle, sheep and goat inocula. Slovak J. Anim. Sci. 2016, 49, 32–43. [Google Scholar]
- Cone, J.W.; van Gelder, A.H.; Bachmann, H. Influence of inoculum source on gas production profiles. Anim. Feed Sci. Technol. 2002, 99, 221–231. [Google Scholar] [CrossRef]
- Mould, F.L.; Morgan, R.; Kliem, K.E.; Krystallidou, E. A review and simplification of the in vitro incubation medium. Anim. Feed Sci. Technol. 2005, 123, 155–172. [Google Scholar] [CrossRef]
- Amanzougarene, Z.; Yuste, S.; Fondevila, M. Fermentation Pattern of Several Carbohydrate Sources Incubated in An in Vitro Semicontinuous System with Inocula from Ruminants Given Either Forage or Concentrate-Based Diets. Animals 2020, 10, 261. [Google Scholar] [CrossRef] [Green Version]
- Borba, A.E.S.; Correia, P.J.A.; Fernandes, J.M.M.; Borba, A.F.R.S. Comparison of three sources of inocula for predicting apparent digestibility of ruminant feedstuffs. Anim. Res. 2001, 50, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Chaudhry, A.S.; Mohamed, R.A.I. Fresh or frozen rumen contents from slaughtered cattle to estimate in vitro degradation of two contrasting feeds. Czech J. Anim. Sci. 2012, 57, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Cornou, C.; Storm, I.M.L.D.; Hindrichsen, I.K.; Worgan, H.; Bakewell, E.; Ruiz, D.R.; Yáñez, A.L.; Tagliapietra, F.; Cattani, M.; Ritz, C.; et al. A ring test of a wireless in vitro gas production system. Anim. Prod. Sci. 2013, 53, 585. [Google Scholar] [CrossRef]
- Patra, A.K.; Yu, Z. Effects of gas composition in headspace and bicarbonate concentrations in media on gas and methane production, degradability, and rumen fermentation using in vitro gas production techniques. J. Dairy Sci. 2013, 96, 4592–4600. [Google Scholar] [CrossRef] [Green Version]
- Ramin, M.; Huhtanen, P. Development of an in vitro method for determination of methane production kinetics using a fully automated in vitro gas system—A modelling approach. Anim. Feed. Sci. Technol. 2012, 174, 190–200. [Google Scholar] [CrossRef]
- Gierus, M.; Schiborra, A.; Südekum, K.H.; Rave, G.; Taube, F. Comparison of gas accumulation profiles of several feeds using manual or automated gas production methods. Anim. Feed Sci. Technol. 2008, 147, 310–325. [Google Scholar] [CrossRef]
- Pirondini, M.; Malagutti, L.; Colombini, S.; Amodeo, P.; Crovetto, G.M. Methane yield from dry and lactating cows diets in the Po Plain (Italy) using an in vitro gas production technique. Ital. J. Anim. Sci. 2012, 11, e61. [Google Scholar] [CrossRef]
- Tagliapietra, F.; Cattani, M.; Hansen, H.H.; Bittante, G.; Schiavon, S. High doses of vitamin E and vitamin C influence in vitro rumen microbial activity. Anim. Feed. Sci. Technol. 2013, 183, 210–214. [Google Scholar] [CrossRef]
- Zhang, J.J.; Li, Y.; Zhou, T.; Xu, D.P.; Zhang, P.; Li, S.; Li, H.B. Bioactivities and Health Benefits of Mushrooms Mainly from China. Molecules 2016, 21, 938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [Green Version]
- Papaefstathiou, E.; Agapiou, A.; Giannopoulos, S.; Kokkinofta, R. Nutritional characterization of carobs and traditional carob products. Food Sci. Nutr. 2018, 6, 2151–2161. [Google Scholar] [CrossRef]
- Vinterhalter, B.; Ninkovic, S.; Kozomara, B.; Vinterhalter, D. Carbohydrate nutrition and anthocyanin accumulation in light grown and etiolated shoot cultures of carob (Ceratonia siliqua L.). Arch. Biol. Sci. 2007, 59, 51–56. [Google Scholar] [CrossRef]
- Benchikh, Y.; Louaileche, H.; George, B.; Merlin, A. Changes in bioactive phytochemical content and in vitro antioxidant activity of carob (Ceratonia siliqua L.) as influenced by fruit ripening. Ind. Crops Prod. 2014, 60, 298–303. [Google Scholar] [CrossRef]
- Wu, Q.L.; Wang, M.; Simon, J.E.; Yu, S.C.; Xiao, P.G.; Ho, C.T. Studies on the Chemical Constituents of Loquat Leaves (Eriobotrya japonica). In Oriental Foods and Herbs; ACS Symposium Series; ACS Publications: Washington, DC, USA, 2003; pp. 292–306. [Google Scholar]
- Ferreres, F.; Gomes, D.; Valentão, P.; Gonçalves, R.; Pio, R.; Chagas, E.A.; Seabra, R.M.; Andrade, P.B. Improved loquat (Eriobotrya japonica Lindl.) cultivars: Variation of phenolics and anti- oxidative potential. Food Chem. 2009, 114, 1019–1027. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Katalinic, V.; Milos, M.; Kulisic, T.; Jukic, M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006, 94, 550–557. [Google Scholar] [CrossRef]
- Katsube, T.; Tabata, H.; Ohta, Y.; Yamasaki, Y.; Anuurad, E.; Shiwaku, K.; Yamane, Y. Screening for Antioxidant Activity in Edible Plant Products: Comparison of Low-Density Lipoprotein Oxidation Assay, DPPH Radical Scavenging Assay, and Folin−Ciocalteu Assay. J. Agric. Food Chem. 2004, 52, 2391–2396. [Google Scholar] [CrossRef]
- Gruz, J.; Ayaz, F.A.; Torun, H.; Strnad, M. Phenolic acid content and radical scavenging activity of extracts from medlar (Mespilus germanica L.) fruit at different stages of ripening. Food Chem. 2011, 124, 271–277. [Google Scholar] [CrossRef]
- Chen, X.H.; Ma, L.H.; Dong, Y.W.; Song, H.; Pu, Y.; Zhou, Q.Y. Evaluation of the differences in phenolic compounds and antioxidant activities of five green asparagus (Asparagus officinalis L.) cultivars. Qual. Assur. Saf. Crops Foods 2017, 9, 479–487. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Szymanowska, D.; Szczepaniak, O.M.; Gramza-Michałowska, A.; Kmiecik, D.; Kulczyński, B.; Szulc, P.; Górnaś, P. Composition of polyphenols of asparagus spears (Asparagus officinalis) and their antioxidant potential. Ciência Rural 2019, 49. [Google Scholar] [CrossRef] [Green Version]
- Di Maro, A.; Pacifico, S.; Fiorentino, A.; Galasso, S.; Gallicchio, M.; Guida, V.; Severino, V.; Monaco, P.; Parente, A. Raviscanina wild asparagus (Asparagus acutifolius L.): A nutritionally valuable crop with antioxidant and antiproliferative properties. Food Res. Int. 2013, 53, 180–188. [Google Scholar] [CrossRef]
- Ferrara, L.; Dosi, R.; Di Maro, A.; Guida, V.; Cefarelli, G.; Pacifico, S.; Mastellone, C.; Fiorentino, A.; Rosati, A.; Parente, A. Nutritional values, metabolic profile and radical scavenging capacities of wild asparagus (A. acutifolius L.). J. Food Comp. Anal. 2011, 24, 326–333. [Google Scholar] [CrossRef]
- Adouni, K.; Júlio, A.; Santos-Buelga, C.; González-Paramás, A.M.; Filipe, P.; Rijo, P.; Lima, S.A.; Reis, S.; Fernandes, Â.; Ferreira, I.C.; et al. Roots and rhizomes of wild Asparagus: Nutritional composition, bioactivity and nanoencapsulation of the most potent extract. Food Biosci. 2022, 45, 101334. [Google Scholar] [CrossRef]
- Dong, T.; Han, R.; Yu, J.; Zhu, M.; Zhang, Y.; Gong, Y.; Li, Z. Anthocyanins accumulation and molecular analysis of correlated genes by metabolome and transcriptome in green and purple asparaguses (Asparagus officinalis L.). Food Chem. 2019, 271, 18–28. [Google Scholar] [CrossRef]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F. Characterisation of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef]
- Medina-Lozano, I.; Bertolín, J.R.; Díaz, A. Nutritional value of commercial and traditional lettuce (Lactuca sativa L.) and wild relatives: Vitamin C and anthocyanin content. Food Chem. 2021, 359, 129864. [Google Scholar] [CrossRef]
- Kim, D.-K. Antioxidative Components from the Aerial Parts of Lactuca scariola L. Arch. Pharmacal Res. 2001, 24, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhang, S.; Yu, L.; Ma, L. Evaluation of antioxidant property and quality of breads containing Auricularia auricula polysaccharide flour. Food Chem. 2007, 101, 1158–1163. [Google Scholar] [CrossRef]
- Kroh, L.W. Caramelisation in food and beverages. Food Chem. 1994, 51, 373–379. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Conde-Aguilera, J.A.; Haro, A.; de la Cueva, S.P.; Rufián-Henares, J.Á. A combined procedure to evaluate the global antioxidant response of bread. J. Cereal Sci. 2010, 52, 239–246. [Google Scholar] [CrossRef]
- Mertens, D.R. Gravimetric determination of amylase-treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: Collaborative study. J. AOAC Int. 2002, 85, 1217–1240. [Google Scholar]
- Žilić, S. Phenolic Compounds of Wheat. Their Content, Antioxidant Capacity and Bioaccessibility. MOJ Food Process. Technol. 2016, 2, 00037. [Google Scholar] [CrossRef]
- Abbas, S.R.; Sabir, S.M.; Ahmad, S.D.; Boligon, A.A.; Athayde, M.L. Phenolic profile, antioxidant potential and DNA damage protecting activity of sugarcane (Saccharum officinarum). Food Chem. 2014, 147, 10–16. [Google Scholar] [CrossRef] [PubMed]








| Feed Resources | Scientific Name | Part Tested |
|---|---|---|
| Wild asparagus | Asparagus aphyllus | Aerial parts |
| Prickly lettuce | Lactuca serriola | Leaves |
| Loquat | Eriobotrya japonica | Leaves |
| Mature carob | Ceratonia siliqua | Pods |
| Maltese bread | Ħobża Maltija * | Crust and crumb |
| Feed Sources | DM | CP | EE | NDF | ADF | Ash |
|---|---|---|---|---|---|---|
| Sheep Feed | 89.50 ± 0.04 | 14.2 ± 0.40 | 3.57 ± 0.03 | 46.64 ± 0.30 | 19.70 ± 0.40 | 9.70 ± 0.20 |
| M_bread | 90.4 ± 0.11 | 6.60 ± 0.15 | 2.90 ± 0.01 | 39.92 ± 0.42 | 4.36 ± 0.25 | 7.31 ± 0.17 |
| W_asparagus | 93.47 ± 0.14 | 8.90 ± 0.16 | 5.24 ± 0.18 | 50.45 ± 1.56 | 46.38 ± 0.53 | 12 ± 0.30 |
| Pr_lettuce | 90.05 ± 0.08 | 12.31 ± 0.18 | 2.31 ± 0.05 | 52.92 ± 0.38 | 38.64 ± 0.34 | 12.24 ± 0.27 |
| Loquat | 91.62 ± 0.09 | 5.70 ± 0.30 | 6.43 ± 0.05 | 36.80 ± 0.91 | 26.77 ± 0.58 | 17.08 ± 0.25 |
| M_carob | 87.75 ± 0 | 6.33 ± 0.07 | 0.01 ± 0 | 56.22 ± 0.32 | 25.80 ± 0.45 | 5.27 ± 0.03 |
| Substrate (S) | a | b | c | a24 | a 48 | 24/48 | 48/a |
|---|---|---|---|---|---|---|---|
| Sheep feed | 134.20 bc | 2.87 | 0.080 | 85.41 b | 121.25 ab | 0.663 b | 0.88 ab |
| Loquat | 113.96 ab | 2.32 | 0.210 | 71.098 b | 99.75 ab | 0.675 b | 0.85 ab |
| M_carob | 152.00 bc | 2.48 | 0.078 | 100.47 ab | 142.54 a | 0.706 ab | 1.00 ab |
| W_asparagus | 74.62 a | 4.17 | 0.080 | 45.86 c | 66.32 b | 0.691 b | 0.88 ab |
| Pr_lettuce | 111.29 ab | 2.64 | 0.066 | 67.31 b | 96.02 ab | 0.700 ab | 0.86 ab |
| M_bread | 183.43 c | 3.64 | 0.159 | 170.26 a | 188.08 ab | 0.960 a | 1.01 a |
| SEM | 8.55 | 0.26 | 0.03 | 9.87 | 10.5 | 0.002 | 0.03 |
| P | 0.007 | ns | ns | <0.001 | <0.001 | 0.005 | 0.016 |
| Feed Sources | Color Index (Abs) | Tint Ratio | Flavonoid Ratio |
|---|---|---|---|
| Sheep feed | 3.02 ± 0.15 | 5.91 ± 0.23 | 0.05 ± 0.002 |
| M_bread | 0.3 ± 0.15 | 0.99 ± 0.013 | 0.23 ± 0.08 |
| W_asparagus | 26.5 ± 1.06 | 4.67 ± 0.39 | 0.09 ± 0.009 |
| Pr_lettuce | 20.2 ± 0.49 | 6.52 ± 0.44 | 0.07 ± 0.004 |
| Loquat | 3.55 ± 0.24 | 1.88 ± 0.041 | 0.03 ± 0.001 |
| M_carob | 1.04 ± 0.05 | 1.85 ± 0.045 | 0.02 ± 0.008 |
| Variables | Tint | %Red | %Yellow | %Blue | Antho | Flav Ratio | DM | Ash | CP | Fat | NDF | ADF | PolyP | DPPH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CI | 0.714 | −0.600 | 0.714 | −0.714 | 1.000 | 0.086 | 0.543 | 0.714 | 0.257 | 0.429 | 0.143 | 0.943 | 0.771 | −0.829 |
| Tint | −0.943 | 1.000 | −1.000 | 0.714 | −0.029 | −0.029 | 0.543 | 0.714 | 0.086 | 0.257 | 0.543 | 0.314 | −0.486 | |
| %Red | −0.943 | 0.943 | −0.600 | 0.086 | 0.257 | −0.257 | −0.771 | 0.200 | −0.543 | −0.486 | −0.143 | 0.257 | ||
| %Yellow | −1.000 | 0.714 | −0.029 | −0.029 | 0.543 | 0.714 | 0.086 | 0.257 | 0.543 | 0.314 | −0.486 | |||
| %Blue | −0.714 | 0.029 | 0.029 | −0.543 | −0.714 | −0.086 | −0.257 | −0.543 | −0.314 | 0.486 | ||||
| Antho | 0.086 | 0.543 | 0.714 | 0.257 | 0.429 | 0.143 | 0.943 | 0.771 | −0.829 | |||||
| Flav Ratio | 0.543 | 0.029 | 0.371 | 0.143 | −0.257 | −0.029 | −0.314 | 0.200 | ||||||
| DM | 0.600 | −0.200 | 0.771 | −0.543 | 0.486 | 0.543 | −0.600 | |||||||
| Ash | −0.029 | 0.657 | −0.429 | 0.600 | 0.771 | −0.886 | ||||||||
| CP | −0.143 | 0.257 | 0.029 | −0.371 | 0.143 | |||||||||
| Fat | −0.771 | 0.257 | 0.543 | −0.714 | ||||||||||
| NDF | 0.314 | −0.086 | 0.257 | |||||||||||
| ADF | 0.829 | −0.771 | ||||||||||||
| PolyP | −0.943 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastorelli, G.; Simeonidis, K.; Faustini, M.; Le Mura, A.; Cavalleri, M.; Serra, V.; Attard, E. Chemical Characterization and In Vitro Gas Production Kinetics of Alternative Feed Resources for Small Ruminants in the Maltese Islands. Metabolites 2023, 13, 762. https://doi.org/10.3390/metabo13060762
Pastorelli G, Simeonidis K, Faustini M, Le Mura A, Cavalleri M, Serra V, Attard E. Chemical Characterization and In Vitro Gas Production Kinetics of Alternative Feed Resources for Small Ruminants in the Maltese Islands. Metabolites. 2023; 13(6):762. https://doi.org/10.3390/metabo13060762
Chicago/Turabian StylePastorelli, Grazia, Kalliroi Simeonidis, Massimo Faustini, Angelo Le Mura, Mariagrazia Cavalleri, Valentina Serra, and Everaldo Attard. 2023. "Chemical Characterization and In Vitro Gas Production Kinetics of Alternative Feed Resources for Small Ruminants in the Maltese Islands" Metabolites 13, no. 6: 762. https://doi.org/10.3390/metabo13060762
APA StylePastorelli, G., Simeonidis, K., Faustini, M., Le Mura, A., Cavalleri, M., Serra, V., & Attard, E. (2023). Chemical Characterization and In Vitro Gas Production Kinetics of Alternative Feed Resources for Small Ruminants in the Maltese Islands. Metabolites, 13(6), 762. https://doi.org/10.3390/metabo13060762