Metabolism Study of Anamorelin, a GHSR1a Receptor Agonist Potentially Misused in Sport, with Human Hepatocytes and LC-HRMS/MS
Abstract
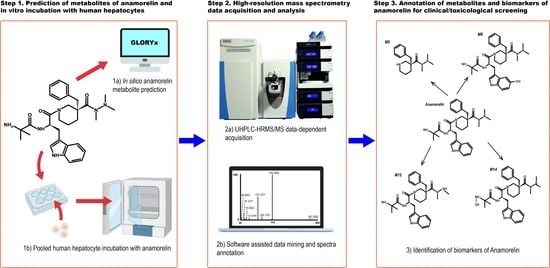
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. In Silico Metabolites Prediction
2.3. Incubation with Pooled Human Hepatocytes
2.4. Sample Preparation
2.5. LC-HRMS/MS Analysis
2.5.1. Liquid Chromatography Conditions
2.5.2. Mass Spectrometry Conditions
2.5.3. Identification of Metabolites
3. Results
3.1. In Silico Prediction
3.2. Fragmentation Pattern
3.3. Metabolite Identification
3.3.1. N-Demethylation
3.3.2. N-Dealkylation (Carboxamide Hydrolysis)
3.3.3. Oxidation
3.3.4. Carboxylation
3.3.5. Glucuronidation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiesing, U. Should Performance-Enhancing Drugs in Sport be Legalized under Medical Supervision? Sports Med. 2011, 41, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Reardon, C.; Creado, S. Drug abuse in athletes. Subs. Abuse Rehabil. 2014, 5, 95–105. [Google Scholar] [CrossRef] [Green Version]
- La Gerche, A.; Brosnan, M.J. Cardiovascular Effects of Performance-Enhancing Drugs. Circulation 2017, 135, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Min, H.; Han, B.; Sung, C.; Park, J.H.; Lee, K.M.; Kim, H.J.; Kim, K.H.; Son, J.; Kwon, O.S.; Lee, J. LC-MS/MS Method for Simultaneous Analysis of Growth Hormone-Releasing Peptides and Secretagogues in Human Urine. Mass Spectrom. Lett. 2016, 7, 55–63. [Google Scholar] [CrossRef] [Green Version]
- World Anti-Doping Agency. Prohibited List 2023. Available online: https://www.wada-ama.org/sites/default/files/2022-09/2023list_en_final_9_september_2022.pdf (accessed on 25 July 2023).
- Wang, G.; Lee, H.M.; Englander, E.; Greeley, G.H. Ghrelin—Not just another stomach hormone. Regul. Pept. 2002, 105, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, G.; Samson, S.L.; Sun, Y. Ghrelin: Much more than a hunger hormone. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 619–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poher, A.L.; Tschöp, M.H.; Müller, T.D. Ghrelin regulation of glucose metabolism. Peptides 2018, 100, 236–242. [Google Scholar] [CrossRef]
- Currow, D.; Maddocks, M.; Cella, D.; Muscaritoli, M. Efficacy of Anamorelin, a Novel Non-Peptide Ghrelin Analogue, in Patients with Advanced Non-Small Cell Lung Cancer (NSCLC) and Cachexia—Review and Expert Opinion. Int. J. Mol. Sci. 2018, 19, 3471. [Google Scholar] [CrossRef] [Green Version]
- Uçaktürk, E.; Başaran, A.A.; Demirel, A.H. Effect of the Mobile Phase Compositions on the Confirmation Analysis of Some Prohibited Substances in Sport by LC–ESI–MS/MS. Chromatographia 2020, 83, 1397–1411. [Google Scholar] [CrossRef]
- Okidono, Y.; Osada, J.; Otsu, K.; Kowase, S.; Aoki, H.; Yumoto, K. Two cases of wide QRS complex tachycardia caused by anamorelin. J. Cardiol. Cases 2022, 26, 212–216. [Google Scholar] [CrossRef]
- Kojima, K.; Furukawa, S.; Ishikawa, T.; Inoue, S. First case report of anamorelin-induced fatal arrhythmia complicated by sinus arrest and refractory ventricular tachycardia. Hear. Case Rep. 2023, 9, 185–189. [Google Scholar] [CrossRef] [PubMed]
- European Medicine Agency. Assessment Report–Aldumiz. Available online: https://www.ema.europa.eu/en/documents/assessment-report/adlumiz-epar-refusal-public-assessment-report_en.pdf (accessed on 25 July 2023).
- Stork, C.; Embruch, G.; Šícho, M.; de Bruyn Kops, C.; Chen, Y.; Svozil, D.; Kirchmair, J. NERDD: A web portal providing access to in silico tools for drug discovery. Bioinformatics 2020, 36, 1291–1292. [Google Scholar] [CrossRef] [PubMed]
- de Bruyn Kops, C.; Šícho, M.; Mazzolari, A.; Kirchmair, J. GLORYx: Prediction of the Metabolites Resulting from Phase 1 and Phase 2 Biotransformations of Xenobiotics. Chem. Res. Toxicol. 2021, 34, 286–299. [Google Scholar] [CrossRef]
- Di Trana, A.; Brunetti, P.; Giorgetti, R.; Marinelli, E.; Zaami, S.; Busardò, F.P.; Carlier, J. In silico prediction, LC-HRMS/MS analysis, and targeted/untargeted data-mining workflow for the profiling of phenylfentanyl in vitro metabolites. Talanta 2021, 235, 122740. [Google Scholar] [CrossRef] [PubMed]
- Birzniece, V. Doping in sport: Effects, harm and misconceptions: Doping in sport. Intern. Med. J. 2015, 45, 239–248. [Google Scholar] [CrossRef]
- Ackley, D.C.; Rockich, K.T.; Baker, T.R. Metabolic Stability Assessed by Liver Microsomes and Hepatocytes. Optimization in Drug Discovery. In Methods in Pharmacology and Toxicology; Humana Press: Totowa, NJ, USA, 2004; pp. 151–162. [Google Scholar] [CrossRef]
- Carlier, J.; Diao, X.; Huestis, M.A. Synthetic cannabinoid BB-22 (QUCHIC): Human hepatocytes metabolism with liquid chromatography-high resolution mass spectrometry detection. J. Pharm. Biomed. Anal. 2018, 157, 27–35. [Google Scholar] [CrossRef]
- Minakata, K.; Hasegawa, K.; Nozawa, H.; Yamagishi, I.; Saitoh, T.; Yoshino, A.; Suzuki, M.; Kitamoto, T.; Suzuki, O.; Watanabe, K. Sensitive quantification of BB-22 and its metabolite BB-22 3-carboxyindole, and characterization of new metabolites in authentic urine and/or serum specimens obtained from three individuals by LC–QTRAP-MS/MS and high-resolution LC–Orbitrap-MS/MS. Forensic Toxicol. 2019, 37, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Busardò, F.P.; Lo Faro, A.F.; Sirignano, A.; Giorgetti, R.; Carlier, J. In silico, in vitro, and in vivo human metabolism of acetazolamide, a carbonic anhydrase inhibitor and common “diuretic and masking agent” in doping. Arch. Toxicol. 2022, 96, 1989–2001. [Google Scholar] [CrossRef]
- Malaca, S.; Bottinelli, C.; Fanton, L.; Cartiser, N.; Carlier, J.; Busardò, F.P. α-Methyltryptamine (α-MT) Metabolite Profiling in Human Hepatocyte Incubations and Postmortem Urine and Blood. Metabolites 2023, 13, 92. [Google Scholar] [CrossRef]
- Li, A.P.; Lu, C.; Brent, J.A.; Pham, C.; Fackett, A.; Ruegg, C.E.; Silbe, P.M. Cryopreserved human hepatocytes: Characterization of drug-metabolizing enzyme activities and applications in higher throughput screening assays for hepatotoxicity, metabolic stability, and drug-drug interaction potential. Chem. Biol. Interact. 1999, 121, 17–35. [Google Scholar] [CrossRef]
- Somers, G.I.; Lindsay, N.; Lowdon, B.M.; Jones, A.E.; Freathy, C.; Ho, S.; Woodrooffe, A.J.M.; Bayliss, M.K.; Manchee, G.R. A Comparison of the Expression and Metabolizing Activities of Phase I and II Enzymes in Freshly Isolated Human Lung Parenchymal Cells and Cryopreserved Human Hepatocytes. Drug Metab. Dispos. 2007, 35, 1797–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita-Tanaka, S.; Yamada, T.; Takayama, K. The landscape of cancer cachexia in advanced non-small cell lung cancer: A narrative review. Transl. Lung Cancer Res. 2023, 12, 168–180. [Google Scholar] [CrossRef] [PubMed]


| ID | Biotransformation | Elemental Composition | RT, min | [M + H]+, m/z | Mass Error, ∆ppm | Diagnostic Productions, m/z | Peak Area at T3h |
|---|---|---|---|---|---|---|---|
| M1 | N-Dealkylation (piperidine) +N-Demethylation +Oxidation (N-methyl) +Dehydrogenation (N-methyl) | C15H21N3O2 | 5.45 | 276.1709 | 0.89 | 82, 91, 174, 202, 276 | 1.2 × 106 |
| M2 | N-Dealkylation (piperidine) +Oxidation (N-methyl) +Dehydrogenation (N-methyl) | C16H23N3O2 | 7.56 | 290.1856 | −2.40 | 82, 91, 174, 202 | 5.2 × 107 |
| M3 | N-Dealkylation (piperidine) +N-Demethylation | C15H23N3O | 8.88 | 262.1907 | −2.63 | 91, 153, 174, 202, 245 | 2.1 × 107 |
| M4 | N-Demethylation +Oxidation (indole) +Glucuronidation | C36H48N6O10 | 11.02 | 725.3513 | 0.83 | 73, 91, 174, 245, 262 | 5.2 × 106 |
| M5 | N-Dealkylation (piperidine) | C16H25N3O | 11.04 | 276.2063 | −2.68 | 75, 91, 174, 202 | 3.3 × 108 |
| M6 | Oxidation (indole) +Glucuronidation | C37H50N6O10 | 12.13 | 739.3676 | 2.00 | 91, 174, 202, 276, 464 | 1.5 × 107 |
| M7 | N-Demethylation +Oxidation (indole) | C30H40N6O4 | 12.78 | 549.3190 | 1.13 | 73, 174, 245, 262, 288 | 3.0 × 107 |
| M8 | Oxidation (indole) | C31H42N6O4 | 14.04 | 563.3348 | 1.37 | 148, 174, 202, 276, 288 | 1.3 × 108 |
| M9 | N-Demethylation +N-Demethylation | C29H38N6O3 | 14.40 | 519.3084 | 1.13 | 73, 174, 262 | 6.1 × 106 |
| M10 | N-Oxidation (dimethylamine) +Oxidation (indole) | C31H42N6O5 | 14.98 | 579.3296 | 1.13 | 75, 148, 174, 202, 276 | 2.1 × 106 |
| M11 | Oxidation (indole) | C31H42N6O4 | 15.05 | 563.3348 | 1.37 | 75, 174, 202, 276, 288 | 2.6 × 107 |
| M12 | N-Demethylation | C30H40N6O3 | 15.33 | 533.3243 | 1.56 | 153, 174, 245, 262 | 3.3 × 108 |
| M13 | N-Demethylation +N-Oxidation (dimethylamine) | C30H40N6O4 | 16.35 | 549.3190 | 1.13 | 73, 174, 187, 245, 262 | 1.1 × 107 |
| Anamorelin (parent) | C31H42N6O3 | 16.41 | 547.3395 | 0.70 | 75, 91, 174, 202, 272, 276 | 5.9 × 109 | |
| M14 | N-Oxidation (dimethylamine) | C31H42N6O4 | 17.46 | 563.3353 | 2.25 | 75, 187, 174, 202, 276, 288 | 1.1 × 108 |
| M15 | Oxidation (Indole) +Carboxylation (dimethylamine) | C31H40N6O6 | 19.58 | 593.3085 | 0.49 | 75, 133, 202, 174, 276 | 4.4 × 106 |
| M16 | N-Demethylation +Carboxylation (dimethylamine) | C30H38N6O5 | 19.81 | 563.2979 | 0.45 | 73, 130, 174, 245, 262 | 7.2 × 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gameli, P.S.; Taoussi, O.; Basile, G.; Carlier, J.; Busardò, F.P. Metabolism Study of Anamorelin, a GHSR1a Receptor Agonist Potentially Misused in Sport, with Human Hepatocytes and LC-HRMS/MS. Metabolites 2023, 13, 949. https://doi.org/10.3390/metabo13080949
Gameli PS, Taoussi O, Basile G, Carlier J, Busardò FP. Metabolism Study of Anamorelin, a GHSR1a Receptor Agonist Potentially Misused in Sport, with Human Hepatocytes and LC-HRMS/MS. Metabolites. 2023; 13(8):949. https://doi.org/10.3390/metabo13080949
Chicago/Turabian StyleGameli, Prince Sellase, Omayema Taoussi, Giuseppe Basile, Jeremy Carlier, and Francesco Paolo Busardò. 2023. "Metabolism Study of Anamorelin, a GHSR1a Receptor Agonist Potentially Misused in Sport, with Human Hepatocytes and LC-HRMS/MS" Metabolites 13, no. 8: 949. https://doi.org/10.3390/metabo13080949
APA StyleGameli, P. S., Taoussi, O., Basile, G., Carlier, J., & Busardò, F. P. (2023). Metabolism Study of Anamorelin, a GHSR1a Receptor Agonist Potentially Misused in Sport, with Human Hepatocytes and LC-HRMS/MS. Metabolites, 13(8), 949. https://doi.org/10.3390/metabo13080949