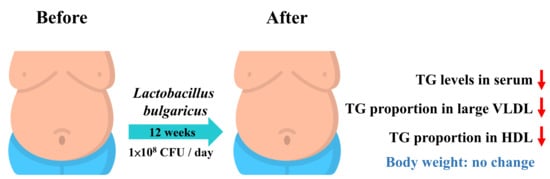
The Efficacy of Lactobacillus delbrueckii ssp. bulgaricus Supplementation in Managing Body Weight and Blood Lipids of People with Overweight: A Randomized Pilot Trial
Abstract
:1. Introduction
2. Methods and Design
2.1. Study Approval
2.2. Participants
2.3. Study Design and Intervention
2.4. Metabolite Quantification and Metabolome Data Analysis
3. Results
3.1. Subject Disposition and Characteristics
3.2. Efficacy Analysis
3.3. Findings of Metabolome Lipid Profiling
3.4. Differential Expression Analysis
3.5. Harms
4. Discussion
5. Limitation and Recommendation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, D.; Heus, P.; van de Wetering, F.T.; van Tienhoven, G.; Verleye, L.; Scholten, R.J. Probiotics for the prevention or treatment of chemotherapy- or radiotherapy-related diarrhoea in people with cancer. Cochrane Database Syst. Rev. 2018, 8, CD008831. [Google Scholar] [CrossRef]
- Meštrović, T.; Matijašić, M.; Perić, M.; Čipčić Paljetak, H.; Barešić, A.; Verbanac, D. The role of gut, vaginal, and urinary microbiome in urinary tract infections: From bench to bedside. Diagnostics 2020, 11, 7. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Bezirtzoglou, E. Probiotics in medicine: A long debate. Front. Immunol. 2020, 11, 2192. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Walker, A.; Haange, S.B.; Lagkouvardos, I.; Liu, Y.; Schmitt-Kopplin, P.; von Bergen, M.; Jehmlich, N.; He, X.; Clavel, T.; et al. Synbiotic-driven improvement of metabolic disturbances is associated with changes in the gut microbiome in diet-induced obese mice. Mol. Metab. 2019, 22, 96–109. [Google Scholar] [CrossRef]
- Singh, R.P.; Shadan, A.; Ma, Y. Biotechnological applications of probiotics: A multifarious weapon to disease and metabolic abnormality. Probiotics Antimicrob. Proteins 2022, 14, 1184–1210. [Google Scholar] [CrossRef]
- Bervoets, L.; Van Hoorenbeeck, K.; Kortleven, I.; Van Noten, C.; Hens, N.; Vael, C.; Goossens, H.; Desager, K.N.; Vankerckhoven, V. Differences in gut microbiota composition between obese and lean children: A cross-sectional study. Gut Pathog. 2013, 5, 10. [Google Scholar] [CrossRef]
- Duan, M.; Wang, Y.; Zhang, Q.; Zou, R.; Guo, M.; Zheng, H. Characteristics of gut microbiota in people with obesity. PLoS ONE 2021, 16, e0255446. [Google Scholar] [CrossRef]
- John, G.K.; Wang, L.; Nanavati, J.; Twose, C.; Singh, R.; Mullin, G. Dietary alteration of the gut microbiome and its impact on weight and fat mass: A systematic review and meta-analysis. Genes 2018, 9, 167. [Google Scholar] [CrossRef]
- Michael, D.R.; Jack, A.A.; Masetti, G.; Davies, T.S.; Loxley, K.E.; Kerry-Smith, J.; Plummer, J.F.; Marchesi, J.R.; Mullish, B.H.; McDonald, J.A.K.; et al. A randomised controlled study shows supplementation of overweight and obese adults with lactobacilli and bifidobacteria reduces bodyweight and improves well-being. Sci. Rep. 2020, 10, 4183. [Google Scholar] [CrossRef]
- Michael, D.R.; Davies, T.S.; Jack, A.A.; Masetti, G.; Marchesi, J.R.; Wang, D.; Mullish, B.H.; Plummer, S.F. Daily supplementation with the Lab4P probiotic consortium induces significant weight loss in overweight adults. Sci. Rep. 2021, 11, 5. [Google Scholar] [CrossRef]
- Jung, S.P.; Lee, K.M.; Kang, J.H.; Yun, S.I.; Park, H.O.; Moon, Y.; Kim, J.Y. Effect of Lactobacillus gasseri BNR17 on overweight and obese adults: A randomized, double-blind clinical trial. Korean J. Fam. Med. 2013, 34, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.; Darimont, C.; Drapeau, V.; Emady-Azar, S.; Lepage, M.; Rezzonico, E.; Ngom-Bru, C.; Berger, B.; Philippe, L.; Ammon-Zuffrey, C.; et al. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Br. J. Nutr. 2014, 111, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Suzumura, E.A.; Bersch-Ferreira, Â.C.; Torreglosa, C.R.; da Silva, J.T.; Coqueiro, A.Y.; Kuntz, M.G.F.; Chrispim, P.P.; Weber, B.; Cavalcanti, A.B. Effects of oral supplementation with probiotics or synbiotics in overweight and obese adults: A systematic review and meta-analyses of randomized trials. Nutr. Rev. 2019, 77, 430–450. [Google Scholar] [CrossRef] [PubMed]
- Widyastuti, Y.; Febrisiantosa, A.; Tidona, F. Health-promoting properties of Lactobacilli in fermented dairy products. Front. Microbiol. 2021, 12, 673890. [Google Scholar] [CrossRef]
- Crovesy, L.; Ostrowski, M.; Ferreira, D.M.T.P.; Rosado, E.L.; Soares-Mota, M. Effect of Lactobacillus on body weight and body fat in overweight subjects: A systematic review of randomized controlled clinical trials. Int. J. Obes. 2017, 41, 1607–1614. [Google Scholar] [CrossRef]
- Lin, Y.K.; Lin, Y.H.; Chiang, C.F.; Yeh, T.M.; Shih, W.L. Lactobacillus delbrueckii subsp. bulgaricus strain TCI904 reduces body weight gain, modulates immune response, improves metabolism and anxiety in high-fat diet-induced obese mice. 3 Biotech 2022, 12, 341. [Google Scholar] [CrossRef]
- Soininen, P.; Kangas, A.J.; Würtz, P.; Suna, T.; Ala-Korpela, M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ. Cardiovasc. Genet. 2015, 8, 192–206. [Google Scholar] [CrossRef]
- Lobstein, T.; Jackson-Leach, R.; Powis, J.; Brinsden, H.; Gray, M. World Obesity Atlas 2023. 2023. Available online: https://www.worldobesityday.org/assets/downloads/World_Obesity_Atlas_2023_Report.pdf (accessed on 27 December 2023).
- Cerdó, T.; García-Santos, J.A.; Bermúdez, M.G.; Campoy, C. The role of probiotics and prebiotics in the prevention and treatment of obesity. Nutrients 2019, 11, 635. [Google Scholar] [CrossRef]
- Park, S.; Ji, Y.; Jung, H.Y.; Park, H.; Kang, J.; Choi, S.H.; Shin, H.; Hyun, C.K.; Kim, K.T.; Holzapfel, W.H. Lactobacillus plantarum HAC01 regulates gut microbiota and adipose tissue accumulation in a diet-induced obesity murine model. Appl. Microbiol. Biotechnol. 2017, 101, 1605–1614. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, B.; Hu, J.; Nie, S.; Xiong, T.; Xie, M. Intervention of five strains of Lactobacillus on obesity in mice induced by a high-fat diet. J. Funct. Foods 2020, 72, 104078. [Google Scholar] [CrossRef]
- Yoo, S.R.; Kim, Y.J.; Park, D.Y.; Jung, U.J.; Jeon, S.M.; Ahn, Y.T.; Huh, C.S.; McGregor, R.; Choi, M.S. Probiotics L. plantarum and L. curvatus in combination alter hepatic lipid metabolism and suppress diet-induced obesity. Obesity 2013, 21, 2571–2578. [Google Scholar] [CrossRef]
- Talayero, B.G.; Sacks, F.M. The role of triglycerides in atherosclerosis. Curr. Cardiol. Rep. 2011, 13, 544–552. [Google Scholar] [CrossRef]
- Feng, R.; Luo, C.; Li, C.; Du, S.; Okekunle, A.P.; Li, Y.; Chen, Y.; Zi, T.; Niu, Y. Free fatty acids profile among lean, overweight and obese non-alcoholic fatty liver disease patients: A case-control study. Lipids Health Dis. 2017, 16, 165. [Google Scholar] [CrossRef]
- Mittendorfer, B.; Yoshino, M.; Patterson, B.W.; Klein, S. VLDL triglyceride kinetics in lean, overweight, and obese men and women. J. Clin. Endocrinol. Metab. 2016, 101, 4151–4160. [Google Scholar] [CrossRef] [PubMed]
- Stadler, J.T.; Marsche, G. Obesity-related changes in high-density lipoprotein metabolism and function. Int. J. Mol. Sci. 2020, 21, 8985. [Google Scholar] [CrossRef]
- Weisweiler, P. Obesity and Lipoproteins. In Biomedical Advances in Aging; Goldstein, A.L., Ed.; GWUMC Department of Biochemistry Annual Spring Symposia; Springer: Boston, MA, USA, 1990. [Google Scholar]
- Huang, J.K.; Lee, H.C. Emerging evidence of pathological roles of very-low-density lipoprotein (VLDL). Int. J. Mol. Sci. 2022, 23, 4300. [Google Scholar] [CrossRef]
- Lee, J.S.; Chang, P.Y.; Zhang, Y.; Kizer, J.R.; Best, L.G.; Howard, B.V. Triglyceride and HDL-C dyslipidemia and risks of coronary heart disease and ischemic stroke by glycemic dysregulation status: The strong heart study. Diabetes Care 2017, 40, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N.; Packard, C.J.; Chapman, M.J.; Borén, J.; Aguilar-Salinas, C.A.; Averna, M.; Ference, B.A.; Gaudet, D.; Hegele, R.A.; Kersten, S.; et al. Triglyceride-rich lipoproteins and their remnants: Metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies—A consensus statement from the European Atherosclerosis Society. Eur. Heart J. 2021, 42, 4791–4806. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Akhmedov, A.; Chen, C.H. Spotlight on very-low-density lipoprotein as a driver of cardiometabolic disorders: Implications for disease progression and mechanistic insights. Front. Cardiovasc. Med. 2022, 9, 993633. [Google Scholar] [CrossRef]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef]
- Hernández, M.A.G.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef]
- Araújo, J.R.; Tazi, A.; Burlen-Defranoux, O.; Vichier-Guerre, S.; Nigro, G.; Licandro, H.; Demignot, S.; Sansonetti, P.J. Fermentation products of commensal bacteria alter enterocyte lipid metabolism. Cell Host Microbe 2020, 27, 358–375.e7. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, H.; Liu, D.; Deng, F. Alterations in the gut microbiota and the efficacy of adjuvant probiotic therapy in liver cirrhosis. Front. Cell. Infect. Microbiol. 2023, 13, 1218552. [Google Scholar] [CrossRef]
- Ma, T.; Shen, X.; Shi, X.; Sakandar, H.A.; Quan, K.; Li, Y.; Jin, H.; Kwok, L.Y.; Zhang, H.; Sun, Z. Targeting gut microbiota and metabolism as the major probiotic mechanism—An evidence-based review. Trends Food Sci. Technol. 2023, 138, 178–198. [Google Scholar] [CrossRef]
- Wang, Z.; Li, L.; Wang, S.; Wei, J.; Qu, L.; Pan, L.; Xu, K. The role of the gut microbiota and probiotics associated with microbial metabolisms in cancer prevention and therapy. Front. Pharmacol. 2022, 13, 1025860. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X.; Zhang, L.; Zheng, K.; Xiong, J.; Li, J.; Cong, C.; Gong, Z.; Mao, J. Effect of probiotics therapy on nonalcoholic fatty liver disease. Comput. Math. Methods Med. 2022, 2022, 7888076. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, T.; Molnár, B.; Németh, D.; Hegyi, P.; Szakács, Z.; Bálint, A.; Garami, A.; Soós, A.; Márta, K.; Solymár, M. Probiotics have beneficial metabolic effects in patients with type 2 diabetes mellitus: A meta-analysis of randomized clinical trials. Sci. Rep. 2020, 10, 11787. [Google Scholar] [CrossRef] [PubMed]
- Woodard, G.A.; Encarnacion, B.; Downey, J.R.; Peraza, J.; Chong, K.; Hernandez-Boussard, T.; Morton, J.M. Probiotic improve outcomes after Roux-en-Y gastric bypass surgery: A prospective randomized trial. J. Gastrointest. Surg. 2009, 13, 1198–1204. [Google Scholar] [CrossRef]
- Kadooka, Y.; Sato, M.; Imaizumi, K.; Ogawa, A.; Ikuyama, K.; Akai, Y.; Okano, M.; Kagoshima, M.; Tsuchida, T. Regulation of abdominal adipocity by probiotics (Lactobaacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur. J. Clin. Nutr. 2010, 64, 636–643. [Google Scholar] [CrossRef]
- Leber, B.; Tripolt, N.J.; Blattl, D.; Eder, M.; Wascher, T.C.; Pieber, T.R.; Stauber, R.; Sourij, H.; Oettl, K.; Stadlbauer, V. The influence of probiotic supplementation on gut permeability in patients with metabolic syndrome: An open label, randomized pilot study. Eur. J. Clin. Nutr. 2012, 66, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Silveira, D.S.C.; Veronez, L.C.; Lopes-Júnior, L.C.; Anatriello, E.; Brunaldi, M.O.; Pereira-da-Silva, G. Lactobacillus bulgaricus inhibits colitis-associated cancer via a negative regulation of intestinal inflammation in azoxymethane/dextran sodium sulfate model. World J. Gastroenterol. 2020, 26, 6782–6794. [Google Scholar] [CrossRef]
- Hong, Y.F.; Lee, Y.D.; Park, J.Y.; Jeon, B.; Jagdish, D.; Jang, S.; Chung, D.K.; Kim, H. Immune regulatory effect of newly isolated Lactobacillus delbrueckii from Indian traditional yogurt. J. Microbiol. Biotechnol. 2015, 25, 1321–1323. [Google Scholar] [CrossRef]
- Wasilewska, E.; Zlotkowska, D.; Wroblewska, B. Yogurt starter cultures of Streptococcus thermophilus and Lactobacillus bulgaricus ameliorate symptoms and modulate the immune response in a mouse model of dextran sulfate sodium-induced colitis. J. Dairy Sci. 2019, 102, 37–53. [Google Scholar] [CrossRef]
- Takahashi, E.; Sawabuchi, T.; Kimoto, T.; Sakai, S.; Kido, H. Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 feeding enhances humoral immune responses, which are suppressed by the antiviral neuraminidase inhibitor oseltamivir in influenza A virus-infected mice. J. Dairy Sci. 2019, 102, 9559–9569. [Google Scholar] [PubMed]
- Mohammadian, T.; Dezfuly, Z.T.; Motlagh, R.G.; Jangaran-Nejad, A.; Hosseini, S.S.; Khaj, H.; Alijani, N. Effect of encapsulated Lactobacillus bulgaricus on innate immune system and hematological parameters in rainbow trout (Oncorhynchus mykiss), post-administration of Pb. Probiotics Antimicrob. Proteins 2020, 12, 375–388. [Google Scholar] [CrossRef]
- Anatriello, E.; Cunha, M.; Nogueira, J.; Carvalho, J.L.; Sá, A.K.; Miranda, M.; Castro-Faria-Neto, H.; Keller, A.C.; Aimbire, F. Oral feeding of Lactobacillus bulgaricus N45.10 inhibits the lung inflammation and airway remodeling in murine allergic asthma: Relevance to the Th1/Th2 cytokines and STAT6/T-bet. Cell. Immunol. 2019, 341, 103928. [Google Scholar] [CrossRef]
- Ishikawa, H.; Kuno, Y.; Kohda, C.; Sasaki, H.; Nagashima, R.; Iyoda, M. Exopolysaccharides from Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 prevent influenza virus infection and attenuate secondary bacterial infection risk. Lett. Appl. Microbiol. 2022, 74, 632–639. [Google Scholar]
- Liu, M.; Liu, M.; Yang, S.; Shen, C.; Wang, X.; Liu, W.; Guo, Y. Fermented milk of cheese-derived Lactobacillus delbrueckii subsp. bulgaricus displays potentials in alleviating alcohol-induced hepatic injury and gut dysbiosis in mice. Food Res. Int. 2022, 157, 111283. [Google Scholar]
- Moro-García, M.A.; Alonso-Arias, R.; Baltadjieva, M.; Fernández Benítez, C.; Fernández Barrial, M.A.; Díaz Ruisánchez, E.; Alonso Santos, R.; Alvarez Sánchez, M.; Saavedra Miján, J.; López-Larrea, C. Oral supplementation with Lactobacillus delbrueckii subsp. bulgaricus 8481 enhances systemic immunity in elderly subjects. Age 2013, 35, 1311–1326. [Google Scholar] [PubMed]
- Sheikhi, A.; Giti, H.; Heibor, M.R.; Jafarzadeh, A.; Shakerian, M.; Baharifar, N.; Niruzad, F.; Moghaddam, A.S.; Kokhaei, P.; Baghaeifar, M. Lactobacilus Delbrueckii subsp. bulgaricus modulates the secretion of Th1/Th2 and Treg cell-related cytokines by PBMCs from patients with atopic dermatitis. Drug Res. 2017, 67, 724–729. [Google Scholar]
- Hajifaraji, M.; Jahanjou, F.; Abbasalizadeh, F.; Aghamohammadzadeh, N.; Abbasi, M.M.; Dolatkhah, N. Effect of probiotic supplements in women with gestational diabetes mellitus on inflammation and oxidative stress biomarkers: A randomized clinical trial. Asia Pac. J. Clin. Nutr. 2018, 27, 581–591. [Google Scholar] [PubMed]
- Yamamoto, Y.; Saruta, J.; Takahashi, T.; To, M.; Shimizu, T.; Hayashi, T.; Morozumi, T.; Kubota, N.; Kamata, Y.; Makino, S.; et al. Effect of ingesting yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 on influenza virus-bound salivary IgA in elderly residents of nursing homes: A randomized controlled trial. Acta Odontol. Scand. 2019, 77, 517–524. [Google Scholar] [PubMed]
- Hemmi, J.; Makino, S.; Yokoo, T.; Kano, H.; Asami, Y.; Takeda, K.; Suzuki, Y.; Kawai, S.; Nagaoka, I.; Sawaki, K.; et al. Consumption of yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 augments serum antibody titers against seasonal influenza vaccine in healthy adults. Biosci. Microbiota Food Health 2023, 42, 73–80. [Google Scholar]



| Group | Probiotics (n = 18) | Placebo (n = 13) | p-Value |
|---|---|---|---|
| Mean ± SEM | Mean ± SEM | ||
| Age, years | 41.56 ± 2.573 | 41.38 ± 3.526 | 0.9042 |
| Weight, kg | 80.77 ± 3.561 | 78.69 ± 4.337 | 0.5888 |
| Body fat, % | 30.84 ± 1.240 | 32.08 ± 1.845 | 0.6742 |
| BMI | 28.89 ± 0.850 | 29.32 ± 1.499 | 0.8102 |
| Variables | Probiotics (n = 18) | Placebo (n = 13) | ||
|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | p-value b | ||
| Weight (kg) | Pre | 80.77 ± 3.561 | 78.69 ± 4.337 | 0.2944 |
| Post | 80.63 ± 3.559 | 77.97 ± 4.193 | 0.2945 | |
| Change | −0.1389 ± 0.3988 | 0.7231 ± 0.8569 | 0.4443 | |
| p-value a | 0.3660 | 0.2076 | ||
| Fat (%) | Pre | 30.84 ± 1.24 | 32.08 ± 1.845 | 0.3371 |
| Post | 31.27 ± 1.189 | 32.55 ± 1.690 | 0.3444 | |
| Change | 0.4278 ± 0.3195 | 0.4692 ± 0.5088 | 0.2479 | |
| p-value a | 0.0991 | 0.1873 | ||
| BMI (kg/m2) | Pre | 28.89 ± 0.8459 | 29.32 ± 1.499 | 0.4051 |
| Post | 28.90 ± 0.8431 | 28.88 ± 1.291 | 0.3974 | |
| Change | 0.0056 ± 0.1620 | −0.4385 ± 0.4160 | 0.3152 | |
| p-value a | 0.4865 | 0.1563 |
| Variables | Probiotics (n = 16–18) | Placebo (n = 12–13) | ||
|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | p-value b | ||
| ALT (U/L) | Pre | 32.00 ± 4.495 | 31.00 ± 6.454 | 0.7219 |
| Post | 28.12 ± 4.853 | 37.69 ± 7.946 | 0.5161 | |
| Change | −3.882 ± 3.461 | 6.692 ± 6.747 | 0.0738 | |
| p-value a | 0.1392 | 0.1704 | ||
| AST (U/L) | Pre | 24.31 ± 1.886 | 22.38 ± 3.054 | 0.2812 |
| Post | 22.44 ± 2.238 | 25.85 ± 4.366 | 0.8429 | |
| Change | −1.875 ± 1.530 | 3.462 ± 2.043 | 0.0147 * | |
| p-value a | 0.1196 | 0.0580 | ||
| BUN (mg/dL) | Pre | 13.82 ± 0.6765 | 13.17 ± 0.8242 | 0.5034 |
| Post | 12.88 ± 0.8985 | 12.67 ± 0.7914 | 0.9626 | |
| Change | −0.6250 ± 0.7465 | −0.500 ± 0.812 | 0.4533 | |
| p-value a | 0.2078 | 0.2753 | ||
| CRE (mg/dL) | Pre | 0.8241 ± 0.03584 | 0.7962 ± 0.0443 | 0.6006 |
| Post | 0.8775 ± 0.04979 | 0.7915 ± 0.04273 | 0.245 | |
| Change | 0.0475 ± 0.02713 | −0.0046 ± 0.0209 | 0.1131 | |
| p-value a | 0.0502 | 0.4143 | ||
| CHOL (mg/dL) | Pre | 200.7 ± 10.33 | 186.9 ± 11.49 | 0.5578 |
| Post | 204.6 ± 12.42 | 186.3 ± 10.20 | 0.4766 | |
| Change | 3.882 ± 7.086 | −0.6154 ± 3.852 | 0.3610 | |
| p-value a | 0.2957 | 0.4379 | ||
| Glu-AC (mg/dL) | Pre | 109.3 ± 7.834 | 91.77 ± 4.590 | 0.0847 |
| Post | 110.7 ± 9.383 | 91.69 ± 6.466 | 0.2138 | |
| Change | 1.389 ± 2.332 | −0.077 ± 2.962 | 0.2171 | |
| p-value a | 0.2797 | 0.4899 | ||
| HbA1c (%) | Pre | 6.124 ± 0.2559 | 5.800 ± 0.2295 | 0.4476 |
| Post | 6.407 ± 0.3149 | 5.931 ± 0.3175 | 0.1878 | |
| Change | 0.2067 ± 0.0589 | 0.131 ± 0.098 | 0.0703 | |
| p-value a | 0.0017 * | 0.1027 | ||
| Insulin (μIU/mL) | Pre | 14.400 ± 5.303 | 9.443 ± 1.610 | 0.6168 |
| Post | 9.670 ± 1.591 | 9.208 ± 1.439 | 0.9001 | |
| Change | −5.040 ± 4.652 | −0.235 ± 0.943 | 0.0513 | |
| p-value a | 0.1473 | 0.4035 | ||
| HDL-C (mg/dL) | Pre | 48.55 ± 3.820 | 46.82 ± 1.613 | 0.3151 |
| Post | 47.38 ± 3.125 | 46.43 ± 1.798 | 0.3925 | |
| Change | −0.0125 ± 1.362 | −0.392 ± 1.590 | 0.4218 | |
| p-value a | 0.4964 | 0.4046 | ||
| LDL-C (mg/dL) | Pre | 113.0 ± 7.399 | 122.8 ± 12.17 | 0.4925 |
| Post | 119.0 ± 5.992 | 110.8 ± 8.862 | 0.7064 | |
| Change | 6.012 ± 4.366 | −12.04 ± 6.323 | 0.0244 * | |
| p-value a | 0.0937 | 0.0417 * | ||
| TG (mg/dL) | Pre | 220.9 ± 42.18 | 139.6 ± 14.47 | 0.1608 |
| Post | 199.9 ± 40.46 | 177.4 ± 36.55 | 0.6008 | |
| Change | −21.00 ± 11.61 | 37.77 ± 34.12 | 0.0412 * | |
| p-value a | 0.0447 * | 0.1450 | ||
| Alb (g/dL) | Pre | 4.541 ± 0.06700 | 4.415 ± 0.07057 | 0.1031 |
| Post | 4.656 ± 0.3614 | 4.485 ± 0.07412 | 0.1793 | |
| Change | 0.125 ± 0.06423 | 0.0692 ± 0.0624 | 0.3287 | |
| p-value a | 0.0353 * | 0.1445 | ||
| CRP (mg/dL) | Pre | 0.2441 ± 0.04415 | 0.2577 ± 0.07466 | 0.6753 |
| Post | 0.2231 ± 0.04520 | 0.1800 ± 0.04403 | 0.6594 | |
| Change | −0.0200 ± 0.03423 | −0.0777 ± 0.0440 | 0.0933 | |
| p-value a | 0.2839 | 0.0513 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, P.-Y.; Yu, Y.-C.; Pan, Y.-C.; Dai, Y.-H.; Yang, J.-C.; Huang, K.-C.; Wu, Y.-C. The Efficacy of Lactobacillus delbrueckii ssp. bulgaricus Supplementation in Managing Body Weight and Blood Lipids of People with Overweight: A Randomized Pilot Trial. Metabolites 2024, 14, 129. https://doi.org/10.3390/metabo14020129
Chu P-Y, Yu Y-C, Pan Y-C, Dai Y-H, Yang J-C, Huang K-C, Wu Y-C. The Efficacy of Lactobacillus delbrueckii ssp. bulgaricus Supplementation in Managing Body Weight and Blood Lipids of People with Overweight: A Randomized Pilot Trial. Metabolites. 2024; 14(2):129. https://doi.org/10.3390/metabo14020129
Chicago/Turabian StyleChu, Pei-Yi, Ying-Chun Yu, Yi-Cheng Pan, Yun-Hao Dai, Juan-Cheng Yang, Kuo-Chin Huang, and Yang-Chang Wu. 2024. "The Efficacy of Lactobacillus delbrueckii ssp. bulgaricus Supplementation in Managing Body Weight and Blood Lipids of People with Overweight: A Randomized Pilot Trial" Metabolites 14, no. 2: 129. https://doi.org/10.3390/metabo14020129
APA StyleChu, P. -Y., Yu, Y. -C., Pan, Y. -C., Dai, Y. -H., Yang, J. -C., Huang, K. -C., & Wu, Y. -C. (2024). The Efficacy of Lactobacillus delbrueckii ssp. bulgaricus Supplementation in Managing Body Weight and Blood Lipids of People with Overweight: A Randomized Pilot Trial. Metabolites, 14(2), 129. https://doi.org/10.3390/metabo14020129