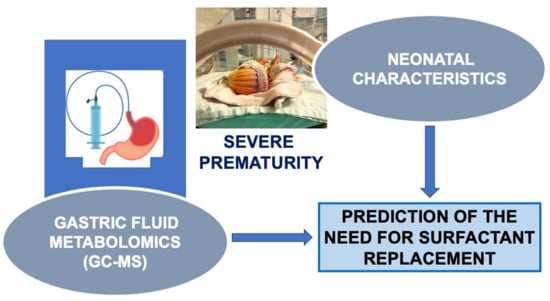
Gastric Fluid Metabolomics Predicting the Need for Surfactant Replacement Therapy in Very Preterm Infants Results of a Case–Control Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Sampling
2.3. Outcomes of Study
2.4. Analytical Methodology
2.4.1. Sample Preparation
2.4.2. GC-MS Analysis
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Study Population
3.2. Differences in Gastric Fluid Samples
3.3. Predictors of the Need for Surfactant Replacement Therapy
3.3.1. Univariate Analysis
3.3.2. Multivariable Analysis
4. Discussion
4.1. Biochemical Role and Origin of Identified Metabolites
4.2. Clinical Parameters to Prognosticate Surfactant Replacement Therapy
4.3. Integration of Metabolomic and Clinical Data
4.4. Advantages and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warren, J.B.; Anderson, J.M. Core Concepts: Respiratory Distress Syndrome. NeoReviews 2009, 10, e351–e361. [Google Scholar] [CrossRef]
- Moya, F.R.; Mazela, J.; Shore, P.M.; Simonson, S.G.; Segal, R.; Simmons, P.D.; Gregory, T.J.; Guardia, C.G.; Varga, J.R.; Finer, N.N.; et al. Prospective Observational Study of Early Respiratory Management in Preterm Neonates Less than 35 Weeks of Gestation. BMC Pediatr. 2019, 19, 147. [Google Scholar] [CrossRef]
- Guinsburg, R.; de Almeida, M.F.B.; de Castro, J.S.; Silveira, R.C.; de Siqueira Caldas, J.P.; Fiori, H.H.; do Vale, M.S.; Abdallah, V.O.S.; Cardoso, L.E.M.B.; Alves Filho, N.; et al. Death or Survival with Major Morbidity in VLBW Infants Born at Brazilian Neonatal Research Network Centers. J. Matern.-Fetal Neonatal Med. 2016, 29, 1005–1009. [Google Scholar] [CrossRef]
- Jobe, A.H. What Is RDS in 2012? Early Hum. Dev. 2012, 88 (Suppl. S2), S42–S44. [Google Scholar] [CrossRef]
- Dargaville, P.A.; Aiyappan, A.; De Paoli, A.G.; Dalton, R.G.B.; Kuschel, C.A.; Kamlin, C.O.; Orsini, F.; Carlin, J.B.; Davis, P.G. Continuous Positive Airway Pressure Failure in Preterm Infants: Incidence, Predictors and Consequences. Neonatology 2013, 104, 8–14. [Google Scholar] [CrossRef]
- Sweet, D.G.; Carnielli, V.P.; Greisen, G.; Hallman, M.; Klebermass-Schrehof, K.; Ozek, E.; Te Pas, A.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome: 2022 Update. Neonatology 2023, 120, 3–23. [Google Scholar] [CrossRef]
- Rodriguez-Fanjul, J.; Jordan, I.; Balaguer, M.; Batista-Muñoz, A.; Ramon, M.; Bobillo-Perez, S. Early Surfactant Replacement Guided by Lung Ultrasound in Preterm Newborns with RDS: The ULTRASURF Randomised Controlled Trial. Eur. J. Pediatr. 2020, 179, 1913–1920. [Google Scholar] [CrossRef]
- Autilio, C. Techniques to Evaluate Surfactant Activity for a Personalized Therapy of RDS Neonates. Biomed. J. 2021, 44, 671–677. [Google Scholar] [CrossRef]
- De Luca, D.; Autilio, C.; Pezza, L.; Shankar-Aguilera, S.; Tingay, D.G.; Carnielli, V.P. Personalized Medicine for the Management of RDS in Preterm Neonates. Neonatology 2021, 118, 127–138. [Google Scholar] [CrossRef]
- Le Gouellec, A.; Plazy, C.; Toussaint, B. What Clinical Metabolomics Will Bring to the Medicine of Tomorrow. Front. Anal. Sci. 2023, 3, 1142606. [Google Scholar] [CrossRef]
- Baraldi, E.; Giordano, G.; Stocchero, M.; Moschino, L.; Zaramella, P.; Tran, M.R.; Carraro, S.; Romero, R.; Gervasi, M.T. Untargeted Metabolomic Analysis of Amniotic Fluid in the Prediction of Preterm Delivery and Bronchopulmonary Dysplasia. PLoS ONE 2016, 11, e0164211. [Google Scholar] [CrossRef]
- Besiri, K.; Begou, O.; Deda, O.; Bataka, E.; Nakas, C.; Gika, H.; Kontou, A.; Agakidou, E.; Sarafidis, K. A Cohort Study of Gastric Fluid and Urine Metabolomics for the Prediction of Survival in Severe Prematurity. Metabolites 2023, 13, 708. [Google Scholar] [CrossRef]
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Plavka, R.; Saugstad, O.D.; Simeoni, U.; Speer, C.P.; Vento, M.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2016 Update. Neonatology 2017, 111, 107–125. [Google Scholar] [CrossRef]
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Te Pas, A.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; Simeoni, U.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2019 Update. Neonatology 2019, 115, 432–450. [Google Scholar] [CrossRef]
- Fabiano, A.; Gazzolo, D.; Zimmermann, L.J.I.; Gavilanes, A.W.D.; Paolillo, P.; Fanos, V.; Caboni, P.; Barberini, L.; Noto, A.; Atzori, L. Metabolomic Analysis of Bronchoalveolar Lavage Fluid in Preterm Infants Complicated by Respiratory Distress Syndrome: Preliminary Results. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2011, 24 (Suppl. S2), 55–58. [Google Scholar] [CrossRef]
- Giambelluca, S.; Verlato, G.; Simonato, M.; Vedovelli, L.; Bonadies, L.; Najdekr, L.; Dunn, W.B.; Carnielli, V.P.; Cogo, P. Chorioamnionitis Alters Lung Surfactant Lipidome in Newborns with Respiratory Distress Syndrome. Pediatr. Res. 2021, 90, 1039–1043. [Google Scholar] [CrossRef]
- Christopoulou, I.; Kostopoulou, E.; Matzarapi, K.; Chasapi, S.A.; Spyroulias, G.A.; Varvarigou, A. Identification of Novel Biomarkers in Late Preterm Neonates with Respiratory Distress Syndrome (RDS) Using Urinary Metabolomic Analysis. Metabolites 2023, 13, 644. [Google Scholar] [CrossRef]
- Widström, A.M.; Christensson, K.; Ransjö-Arvidson, A.B.; Matthiesen, A.S.; Winberg, J.; Uvnäs-Moberg, K. Gastric Aspirates of Newborn Infants: pH, Volume and Levels of Gastrin- and Somatostatin-like Immunoreactivity. Acta Paediatr. Scand. 1988, 77, 502–508. [Google Scholar] [CrossRef]
- Scott, C.R.; Teng, C.C.; Sagerson, R.N.; Nelson, T. Amino Acids in Amniotic Fluid: Changes in Concentrations during the First Half of Pregnancy. Pediatr. Res. 1972, 6, 659–663. [Google Scholar] [CrossRef]
- Sano, M.; Nagura, H.; Ueno, S.; Nakashima, A. Amino Acid Composition of Amniotic Fluid during the Perinatal Period Reflects Mother’s Fat and Carbohydrate Intake. Nutrients 2021, 13, 2136. [Google Scholar] [CrossRef]
- Holeček, M. Serine Metabolism in Health and Disease and as a Conditionally Essential Amino Acid. Nutrients 2022, 14, 1987. [Google Scholar] [CrossRef]
- de Paz-Lugo, P.; Lupiáñez, J.A.; Meléndez-Hevia, E. High Glycine Concentration Increases Collagen Synthesis by Articular Chondrocytes in Vitro: Acute Glycine Deficiency Could Be an Important Cause of Osteoarthritis. Amino Acids 2018, 50, 1357–1365. [Google Scholar] [CrossRef]
- Ghosh, S. Metabolomic Studies for Metabolic Alterations Induced by Non-Steroidal Anti-Inflammatory Drugs: Mini Review. Biomolecules 2021, 11, 1456. [Google Scholar] [CrossRef]
- Mao, X.; Zeng, X.; Qiao, S.; Wu, G.; Li, D. Specific Roles of Threonine in Intestinal Mucosal Integrity and Barrier Function. Front. Biosci. Elite Ed. 2011, 3, 1192–1200. [Google Scholar] [CrossRef]
- Metwaly, S.; Côté, A.; Donnelly, S.J.; Banoei, M.M.; Lee, C.H.; Andonegui, G.; Yipp, B.G.; Vogel, H.J.; Fiehn, O.; Winston, B.W. ARDS Metabolic Fingerprints: Characterization, Benchmarking, and Potential Mechanistic Interpretation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L79–L90. [Google Scholar] [CrossRef]
- Vettore, L.A.; Westbrook, R.L.; Tennant, D.A. Proline Metabolism and Redox; Maintaining a Balance in Health and Disease. Amino Acids 2021, 53, 1779–1788. [Google Scholar] [CrossRef]
- Agassandian, M.; Mallampalli, R.K. Surfactant Phospholipid Metabolism. Biochim. Biophys. Acta 2013, 1831, 612–625. [Google Scholar] [CrossRef]
- Liu, G.; Summer, R. Cellular Metabolism in Lung Health and Disease. Annu. Rev. Physiol. 2019, 81, 403–428. [Google Scholar] [CrossRef]
- Cools, P. The Role of Escherichia Coli in Reproductive Health: State of the Art. Res. Microbiol. 2017, 168, 892–901. [Google Scholar] [CrossRef]
- Hindson, V.J. Serine Acetyltransferase of Escherichia Coli: Substrate Specificity and Feedback Control by Cysteine. Biochem. J. 2003, 375, 745–752. [Google Scholar] [CrossRef]
- Raschetti, R.; Centorrino, R.; Letamendia, E.; Benachi, A.; Marfaing-Koka, A.; De Luca, D. Estimation of Early Life Endogenous Surfactant Pool and CPAP Failure in Preterm Neonates with RDS. Respir. Res. 2019, 20, 75. [Google Scholar] [CrossRef]
- Bell, E.F.; Hintz, S.R.; Hansen, N.I.; Bann, C.M.; Wyckoff, M.H.; DeMauro, S.B.; Walsh, M.C.; Vohr, B.R.; Stoll, B.J.; Carlo, W.A.; et al. Mortality, In-Hospital Morbidity, Care Practices, and 2-Year Outcomes for Extremely Preterm Infants in the US, 2013–2018. JAMA 2022, 327, 248–263. [Google Scholar] [CrossRef]
- Bhandari, V.; Black, R.; Gandhi, B.; Hogue, S.; Kakkilaya, V.; Mikhael, M.; Moya, F.; Pezzano, C.; Read, P.; Roberts, K.D.; et al. RDS-NExT Workshop: Consensus Statements for the Use of Surfactant in Preterm Neonates with RDS. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2023, 43, 982–990. [Google Scholar] [CrossRef]
- Jang, W.; Choi, Y.S.; Kim, J.Y.; Yon, D.K.; Lee, Y.J.; Chung, S.-H.; Kim, C.Y.; Yeo, S.G.; Lee, J. Artificial Intelligence-Driven Respiratory Distress Syndrome Prediction for Very Low Birth Weight Infants: Korean Multicenter Prospective Cohort Study. J. Med. Internet Res. 2023, 25, e47612. [Google Scholar] [CrossRef]
- Acharjee, A.; Hazeldine, J.; Bazarova, A.; Deenadayalu, L.; Zhang, J.; Bentley, C.; Russ, D.; Lord, J.M.; Gkoutos, G.V.; Young, S.P.; et al. Integration of Metabolomic and Clinical Data Improves the Prediction of Intensive Care Unit Length of Stay Following Major Traumatic Injury. Metabolites 2021, 12, 29. [Google Scholar] [CrossRef]
- Rhee, E.P.; Clish, C.B.; Ghorbani, A.; Larson, M.G.; Elmariah, S.; McCabe, E.; Yang, Q.; Cheng, S.; Pierce, K.; Deik, A.; et al. A Combined Epidemiologic and Metabolomic Approach Improves CKD Prediction. J. Am. Soc. Nephrol. 2013, 24, 1330. [Google Scholar] [CrossRef]
- Ramaswamy, V.V.; Bandyopadhyay, T.; Abiramalatha, T.; Pullattayil S, A.K.; Szczapa, T.; Wright, C.J.; Roehr, C.C. Clinical Decision Thresholds for Surfactant Administration in Preterm Infants: A Systematic Review and Network Meta-Analysis. EClinicalMedicine 2023, 62, 102097. [Google Scholar] [CrossRef]

| Cases (n = 43) | Controls (n = 30) | p-Value * | |
|---|---|---|---|
| Gestational age (weeks) | 28 (4.0) | 30 (2.0) | 0.01 |
| Birth weight (g) | 1120 (670) | 1240 (338) | 0.036 |
| Sex (male) | 18 (41.9) | 11 (36.7) | 0.655 |
| SGA | 3 (7) | 3 (10) | 0.644 |
| Multiple gestation | 13 (30.2) | 11 (36.7) | 0.565 |
| Maternal age (years) | 32 (10.0) | 35 (9.0) | 0.243 |
| Prenatal steroids (any) | 41 (95.3) | 29 (96.7) | 0.780 |
| Maternal MgSO4 administration | 15 (34.9) | 7 (23.3) | 0.206 |
| Chorioamnionitis (clinical or histological) | 22 (51.2) | 19 (63.3) | 0.302 |
| Mode of delivery-CS | 37 (88.1) | 27 (90) | 0.80 |
| Apgar score 1 min (median, IQR) Apgar score 5 min (median, IQR) | 7.0 (4.5) 8.0 (1.0) | 7.0 (1.0) 8.5 (1.0) | 0.038 0.024 |
| Hypertension/pregnancy-induced hypertension | 5 (11.9) | 2 (6.9) | 0.487 |
| Intubation in the DR | 21 (48.8) | 3 (10) | <0.001 |
| IMV during the first 3 DOL | 31 (72.1) | 4 (13.3) | <0.001 |
| Confirmed EOS | 1 (2.3) | 0 | 0.400 |
| Metabolite | Group | Mean | SD | SE | Q 0.25 | Q 0.50 | Q 0.75 | p-Value * | AUC (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| L-proline | Cases Controls | 2.58 1.74 | 2.19 2.52 | 0.33 0.46 | 1.17 0.45 | 2.21 1.12 | 3.28 2.03 | 0.018 | 0.66 (0.53–0.79) |
| L-glycine | Cases Controls | 6.05 3.39 | 5.35 3.73 | 0.81 0.68 | 2.67 0.63 | 4.50 2.59 | 8.80 5.07 | 0.036 | 0.64 (0.51–0.77) |
| Acetyl-L-serine | Cases Controls | 6.34 3.52 | 5.62 3.80 | 0.85 0.69 | 2.88 0.68 | 4.65 2.73 | 8.97 5.31 | 0.036 | 0.65 (0.52–0.78) |
| L-threonine | Cases Controls | 2.21 1.34 | 2.16 1.36 | 0.33 0.24 | 1.01 0.67 | 1.42 1.07 | 2.38 1.44 | 0.038 | 0.64 (0.51–0.76) |
| Variable | Univariate | Multivariate * | ||||
|---|---|---|---|---|---|---|
| OR | p-Value | 95% CI | OR | p-Value | 95% CI | |
| Gestational age (weeks) | 0.70 | 0.009 | 0.54–0.91 | |||
| Apgar score at 1 min 5 min | 0.70 0.45 | 0.022 0.026 | 0.51–0.94 0.22–0.91 | |||
| Intubation in the DR | 8.59 | 0.002 | 2.26–32.62 | 8.12 | 0.003 | 2.07–31.84 |
| L-glycine | 1.15 | 0.029 | 1.01–1.30 | |||
| Acetyl-L-serine | 1.14 | 0.027 | 1.01–1.29 | 1.13 | 0.045 | 1.01–1.27 |
| L-proline | 1.20 | 0.146 | 0.93–1.54 | |||
| L-Threonine | 1.36 | 0.076 | 0.96–1.94 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besiri, K.; Begou, O.; Lallas, K.; Kontou, A.; Agakidou, E.; Deda, O.; Gika, H.; Verykouki, E.; Sarafidis, K. Gastric Fluid Metabolomics Predicting the Need for Surfactant Replacement Therapy in Very Preterm Infants Results of a Case–Control Study. Metabolites 2024, 14, 196. https://doi.org/10.3390/metabo14040196
Besiri K, Begou O, Lallas K, Kontou A, Agakidou E, Deda O, Gika H, Verykouki E, Sarafidis K. Gastric Fluid Metabolomics Predicting the Need for Surfactant Replacement Therapy in Very Preterm Infants Results of a Case–Control Study. Metabolites. 2024; 14(4):196. https://doi.org/10.3390/metabo14040196
Chicago/Turabian StyleBesiri, Konstantia, Olga Begou, Konstantinos Lallas, Angeliki Kontou, Eleni Agakidou, Olga Deda, Helen Gika, Eleni Verykouki, and Kosmas Sarafidis. 2024. "Gastric Fluid Metabolomics Predicting the Need for Surfactant Replacement Therapy in Very Preterm Infants Results of a Case–Control Study" Metabolites 14, no. 4: 196. https://doi.org/10.3390/metabo14040196
APA StyleBesiri, K., Begou, O., Lallas, K., Kontou, A., Agakidou, E., Deda, O., Gika, H., Verykouki, E., & Sarafidis, K. (2024). Gastric Fluid Metabolomics Predicting the Need for Surfactant Replacement Therapy in Very Preterm Infants Results of a Case–Control Study. Metabolites, 14(4), 196. https://doi.org/10.3390/metabo14040196