Assessment of l-Asparaginase Pharmacodynamics in Mouse Models of Cancer
Abstract
:1. Introduction
2. Results
2.1. Optimization of Amino Acid Acquisition Parameters and ASNase Activity Quenching
2.2. Accuracy, Precision, Recovery, Normalized Matrix Factor, and FTS Assessments
2.3. Pharmacodynamics of ASNase in NOD.Cg-PRKDC(scid) IL2RG(tm1Wjl) (NSG) Mice
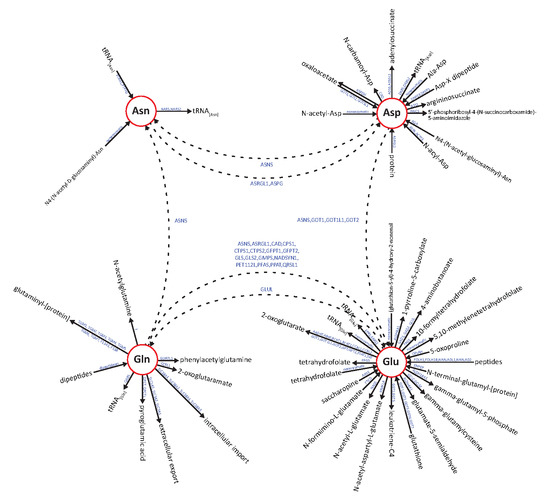
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Equipment and Consumables
4.3. Optimization of Experimental Conditions for the Effective Quenching of ASNase Activity
4.4. Dialysis of Mouse Whole Blood
4.5. Preparation of Amino Acid Stock, Combined Intermediate, and Calibrator Spiking Solutions
4.6. Preparation of Stable-Isotope Labeled Internal Standard Stock and Working Internal Standard Solutions
4.7. Sample Extraction Procedure
4.8. Sample Reconstitution
4.9. Liquid Chromatography/Mass Spectrometry Conditions
4.10. Study Design
4.11. Preparation of Sample Extraction Tubes
4.12. Collection, ASNase Activity Quenching, and Extraction of Mouse Whole Blood Study Samples
4.13. Quantitative Analysis Workflow
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Clementi, A. La désamidation enzymatique de l’asparagine chez les différentes espéces animales et la signification physio logique de sa presence dans l’organisme. Arch. Int. Physiol. 1922, 19, 369–398. [Google Scholar] [CrossRef]
- Kidd, J.G. Regression of transplated lymphomas induced in vivo by means of normal guinea pig serum. J. Exp. Med. 1953, 98, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Broome, J.D. Evidence that the l-asparaginase of guinea pig serum is responsible for its antilymphoma effects. J. Exp. Med. 1963, 118, 99–120. [Google Scholar] [CrossRef] [PubMed]
- Avramis, V.I. Asparaginases: Biochemical pharmacology and modes of drug resistance. Anticancer Res. 2012, 32, 2423–2438. [Google Scholar]
- Aslanian, A.M.; Fletcher, B.S.; Kilberg, M.S. Asparagine synthetase expression alone is sufficient to induce l-asparaginase resistance in molt-4 human leukaemia cells. Biochem. J. 2001, 357, 321–328. [Google Scholar] [CrossRef]
- Lorenzi, P.L.; Reinhold, W.C.; Rudelius, M.; Gunsior, M.; Shankavaram, U.; Bussey, K.J.; Scherf, U.; Eichler, G.S.; Martin, S.E.; Chin, K.; et al. Asparagine synthetase as a causal, predictive biomarker for l-asparaginase activity in ovarian cancer cells. Mol. Cancer Ther. 2006, 5, 2613–2623. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, P.L.; Llamas, J.; Gunsior, M.; Ozbun, L.; Reinhold, W.C.; Varma, S.; Ji, H.; Kim, H.; Hutchinson, A.A.; Kohn, E.C.; et al. Asparagine synthetase is a predictive biomarker of l-asparaginase activity in ovarian cancer cell lines. Mol. Cancer Ther. 2008, 7, 3123–3128. [Google Scholar] [CrossRef]
- Chan, W.K.; Lorenzi, P.L.; Anishkin, A.; Purwaha, P.; Rogers, D.M.; Sukharev, S.; Rempe, S.B.; Weinstein, J.N. The glutaminase activity of l-asparaginase is not required for anticancer activity against asns-negative cells. Blood 2014, 123, 3596–3606. [Google Scholar] [CrossRef] [PubMed]
- Story, M.D.; Voehringer, D.W.; Stephens, L.C.; Meyn, R.E. l-asparaginase kills lymphoma cells by apoptosis. Cancer Chemother. Pharmacol. 1993, 32, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Bussolati, O.; Belletti, S.; Uggeri, J.; Gatti, R.; Orlandini, G.; Dall’Asta, V.; Gazzola, G.C. Characterization of apoptotic phenomena induced by treatment with l-asparaginase in NIH3T3 cells. Exp. Cell Res. 1995, 220, 283–291. [Google Scholar] [CrossRef]
- Ueno, T.; Ohtawa, K.; Mitsui, K.; Kodera, Y.; Hiroto, M.; Matsushima, A.; Inada, Y.; Nishimura, H. Cell cycle arrest and apoptosis of leukemia cells induced by l-asparaginase. Leukemia 1997, 11, 1858–1861. [Google Scholar] [CrossRef] [PubMed]
- Fumarola, C.; Zerbini, A.; Guidotti, G.G. Glutamine deprivation-mediated cell shrinkage induces ligand-independent cd95 receptor signaling and apoptosis. Cell Death Differ. 2001, 8, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Holleman, A.; den Boer, M.L.; Kazemier, K.M.; Janka-Schaub, G.E.; Pieters, R. Resistance to different classes of drugs is associated with impaired apoptosis in childhood acute lymphoblastic leukemia. Blood 2003, 102, 4541–4546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marini, B.L.; Perissinotti, A.J.; Bixby, D.L.; Brown, J.; Burke, P.W. Catalyzing improvements in all therapy with asparaginase. Blood Rev. 2017, 31, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Asselin, B.L.; Lorenson, M.Y.; Whitin, J.C.; Coppola, D.J.; Kende, A.S.; Blakley, R.L.; Cohen, H.J. Measurement of serum l-asparagine in the presence of l-asparaginase requires the presense of and l-asparaginase inhibitor. Cancer Res. 1991, 51, 6568–6573. [Google Scholar] [PubMed]
- Gentili, D.; Zucchetti, M.; Conter, V.; Masera, G.; D’Incalci, M. Determination of l-asparagine in biological samples in the presence of l-asparaginase. J. Chromatogr. B 1994, 657, 47–52. [Google Scholar] [CrossRef]
- Anishkin, A.; Vanegas, J.M.; Rogers, D.M.; Lorenzi, P.L.; Chan, W.K.; Purwaha, P.; Weinstein, J.N.; Sukharev, S.; Rempe, S.B. Catalytic role of the substrate defines specificity of therapeutic l-asparaginase. J. Mol. Biol. 2015, 427, 2867–2885. [Google Scholar] [CrossRef] [PubMed]
- Purwaha, P.; Lorenzi, P.L.; Silva, L.P.; Hawke, D.H.; Weinstein, J.N. Targeted metabolomic analysis of amino acid response to l-asparaginase in adherent cells. Metabolomics 2014, 10, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, D.S.; Park, J.R.; Thomson, B.G.; Felgenhauer, J.L.; Holcenberg, J.S.; Panosyan, E.H.; Avramis, V.I. Asparaginase pharmacokinetics after intensive polyethylene glycol-conjugated l-asparaginase therapy for children with relapsed acute lymphoblastic leukemia. Clin. Cancer Res. 2004, 10, 5335–5341. [Google Scholar] [CrossRef]
- Pieters, R.; Hunger, S.P.; Boos, J.; Rizzari, C.; Silverman, L.; Baruchel, A.; Goekbuget, N.; Schrappe, M.; Pui, C.-H. l-asparaginase treatment in acute lymphoblastic leukemia: A focus on erwinia asparaginase. Cancer 2011, 117, 238–249. [Google Scholar] [CrossRef]
- Appel, I.M.; Kazemier, K.M.; Boos, J.; Lanvers, C.; Huijmans, J.; Veerman, A.J.P.; van Wering, E.; den Boer, M.L.; Pieters, R. Pharmacokinetic, pharmacodynamic and intracellular effects of peg-asparaginase in newly diagnosed childhood acute lymphoblastic leukemia: Results from a single agent window study. Leukemia 2008, 22, 1665–1679. [Google Scholar] [CrossRef] [PubMed]
- Panetta, J.C.; Gajjar, A.; Hijiya, N.; Hak, L.J.; Cheng, C.; Liu, W.; Pui, C.-H.; Relling, M.V. Comparison of native E. coli and peg asparaginase pharmacokinetics and pharmacodynamics in pediatric acute lymphoblastic leukemia. Clin. Pharmacol. Ther. 2009, 86, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Jarrar, M.; Gaynon, P.S.; Periclou, A.P.; Fu, C.; Harris, R.E.; Stram, D.; Altman, A.; Bostrom, B.; Breneman, J.; Steele, D.; et al. Asparagine depletion after pegylated E. coli asparaginase treatment and induction outcome in children with acute lymphoblastic leukemia in first bone marrow relapse: A children’s oncology group study (CCG-1941). Pediatr. Blood Cancer 2006, 47, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.H.; Pieters, R.; Kaspers, G.J.; te Loo, D.M.; Bierings, M.B.; van den Bos, C.; Kollen, W.J.; Hop, W.C.; Lanvers-Kaminsky, C.; Relling, M.V.; et al. A prospective study on drug monitoring of pegasparaginase and erwinia asparaginase and asparaginase antibodies in pediatric acute lymphoblastic leukemia. Blood 2014, 123, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
- Gentili, D.; Conter, V.; Rizzari, C.; Tschuemperlin, B.; Zucchetti, M.; Orlandoni, D.; D’Incalci, M.; Masera, G. l-asparagine depletion in plasma and cerebro-spinal fluid of children with acute lymphoblastic leukemia during subsequent exposures to erwinia l-asparaginase. Ann. Oncol. 1996, 7, 725–730. [Google Scholar] [CrossRef]
- Rotoli, B.M.; Uggeri, J.; Dall’Asta, V.; Visigalli, R.; Barilli, A.; Gatti, R.; Orlandini, G.; Gazzola, G.C.; Bussolati, O. Inhibition of glutamine synthetase triggers apoptosis in asparaginase-resistant cells. Cell Physiol. Biochem. 2005, 15, 281–292. [Google Scholar] [CrossRef]
- Prinsen, H.C.; Schiebergen-Bronkhorst, B.G.; Roeleveld, M.W.; Jans, J.J.; de Sain-van der Velden, M.G.; Visser, G.; van Hasselt, P.M.; Verhoeven-Duif, N.M. Rapid quantification of underivatized amino acids in plasma by hydrophilic interaction liquid chromatography (HILIC) coupled with tandem mass-spectrometry. J. Inherit. Metab. Dis. 2016, 39, 651–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Compound | SRMa (m/z) | Fragmentor Voltage (V) | Collision Energy (V) |
|---|---|---|---|
| [13C0]-Asn | 133.1 → 74.1 | 45 | 17 |
| [13C4,15N2]-Asn | 133.1 → 74.1 | 45 | 17 |
| [13C0]-Asp | 134.0 → 74.1 | 45 | 13 |
| [13C4,15N1]-Asp | 139.1 → 77.1 | 45 | 13 |
| [13C0]-Gln | 147.1 → 84.1 | 45 | 5 |
| [13C4,15N2]-Gln | 154.1 → 89.1 | 45 | 5 |
| [13C0]-Glu | 148.1 → 84.1 | 45 | 17 |
| [13C4,15N2]-Glu | 154.1 → 89.1 | 45 | 17 |
| [Asn]nominal (µM) | [Asn]mean (µM) a | Accuracy (%RE) b | Precision (%CV) c |
|---|---|---|---|
| 12.0 | 10.6 | −11.8% | 4.78% |
| 200 | 182 | −9.00% | 2.31% |
| 3200 | 3140 | −1.89% | 2.38% |
| [Asp]nominal (µM) | [Asp]mean (µM) a | Accuracy (%RE) b | Precision (%CV) c |
| 12.0 | 14.6 | 21.9% | 7.05% |
| 200 | 214 | 7.16% | 3.75% |
| 3200 | 3155 | 1.41% | 2.57% |
| [Gln]nominal (µM) | [Gln]mean (µM) a | Accuracy (%RE) b | Precision (%CV) c |
| 12.0 | 11.1 | −7.87% | 5.77% |
| 200 | 215 | 7.60% | 2.32% |
| 3200 | 3675 | 14.8% | 2.50% |
| [Glu]nominal (µM) | [Glu]mean (µM) a | Accuracy (%RE) b | Precision (%CV) c |
| 12.0 | 14.4 | 20.0% | 6.13% |
| 200 | 209 | 4.36% | 2.16% |
| 3200 | 3122 | −2.42% | 1.56% |
| [Analyte]/[IS] a | Mean Recovery Asn/Asn-IS b | Mean Recovery Asp/Asp-IS c | Mean Recovery Gln/Gln-IS d | Mean Recovery Glu/Glu-IS e |
|---|---|---|---|---|
| 8.00 µM/100 µM | 94%/98% | 97%/97% | 87%/89% | 96%/99% |
| 1000 µM/100 µM | 96%/96% | 101%/99% | 91%/92% | 100%/100% |
| 4000 µM/100 µM | 98%/98% | 100%/98% | 92%/92% | 99%/99% |
| [Analyte]/[IS] a | Mean NMF Asn/Asn-IS b | Mean NMF Asp/Asp-IS c | Mean NMF Gln/Gln-IS d | Mean NMF Glu/Glu-IS e |
| 8.00 µM/100 µM | 0.971 | 0.966 | 0.956 | 0.967 |
| 1000 µM/100 µM | 1.00 | 0.972 | 0.996 | 0.999 |
| 4000 µM/100 µM | 0.998 | 1.01 | 1.00 | 0.993 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horvath, T.D.; Chan, W.K.; Pontikos, M.A.; Martin, L.A.; Du, D.; Tan, L.; Konopleva, M.; Weinstein, J.N.; Lorenzi, P.L. Assessment of l-Asparaginase Pharmacodynamics in Mouse Models of Cancer. Metabolites 2019, 9, 10. https://doi.org/10.3390/metabo9010010
Horvath TD, Chan WK, Pontikos MA, Martin LA, Du D, Tan L, Konopleva M, Weinstein JN, Lorenzi PL. Assessment of l-Asparaginase Pharmacodynamics in Mouse Models of Cancer. Metabolites. 2019; 9(1):10. https://doi.org/10.3390/metabo9010010
Chicago/Turabian StyleHorvath, Thomas D., Wai Kin Chan, Michael A. Pontikos, Leona A. Martin, Di Du, Lin Tan, Marina Konopleva, John N. Weinstein, and Philip L. Lorenzi. 2019. "Assessment of l-Asparaginase Pharmacodynamics in Mouse Models of Cancer" Metabolites 9, no. 1: 10. https://doi.org/10.3390/metabo9010010
APA StyleHorvath, T. D., Chan, W. K., Pontikos, M. A., Martin, L. A., Du, D., Tan, L., Konopleva, M., Weinstein, J. N., & Lorenzi, P. L. (2019). Assessment of l-Asparaginase Pharmacodynamics in Mouse Models of Cancer. Metabolites, 9(1), 10. https://doi.org/10.3390/metabo9010010