Feasibility of Localized Metabolomics in the Study of Pancreatic Islets and Diabetes
Abstract
:1. Introduction
2. Results
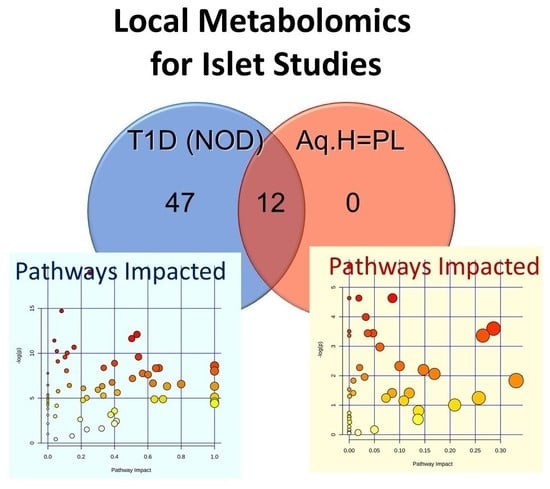
2.1. Non-Targeted Metabolomics in Parallel Local (Aqueous Humor) and Systemic (Plasma) Samples
2.1.1. Non-Targeted Metabolomics in Aqueous Humor Samples
2.1.2. Exploratory Assessment of Diabetes-Induced Changes in the Metabolome
2.2. Representative Findings from A Longitudinal NOD Study
2.2.1. Metabolic Changes in Plasma of T1D Progressor Versus Non-Progressor NOD Mice
2.2.2. Metabolic Pathways Affected by Hyperglycemia Onset in NOD Mice
2.3. Gender-Specific Differences in the Metabolome
3. Discussion
4. Materials and Methods
4.1. Animal Care and Treatment
4.2. Sample Collection
4.3. GC-MS-Based Metabolomics Analysis
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Data Availability
Funding
Conflicts of Interest
References
- Hallgreen, C.E.; Korsgaard, T.V.; Hansen, R.N.; Colding-Jørgensen, M. The glucose-insulin control system. In Biosimulation in Drug Development; Bertau, M., Mosekilde, E., Westerhoff, H.V., Eds.; Wiley-VCH: Weinheim, Germany, 2008; pp. 141–196. [Google Scholar]
- Skyler, J.S.; Ricordi, C. Stopping type 1 diabetes: Attempts to prevent or cure type 1 diabetes in man. Diabetes 2011, 60, 1–8. [Google Scholar] [CrossRef]
- Lernmark, Å.; Larsson, H.E. Immune therapy in type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2013, 9, 92–103. [Google Scholar] [CrossRef]
- Skyler, J.S. Prevention and reversal of type 1 diabetes-past challenges and future opportunities. Diabetes Care 2015, 38, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.A.; Roep, B.O.; Posgai, A.; Wheeler, D.C.S.; Peakman, M. The challenge of modulating β-cell autoimmunity in type 1 diabetes. Lancet Diabetes Endocrinol. 2019, 7, 52–64. [Google Scholar] [CrossRef]
- Donath, M.Y.; Hess, C.; Palmer, E. What is the role of autoimmunity in type 1 diabetes? A clinical perspective. Diabetologia 2014, 57, 653–655. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef]
- Burn, P. Type 1 diabetes. Nat. Rev. Drug Discov. 2010, 9, 187–188. [Google Scholar] [CrossRef]
- Dabelea, D.; Mayer-Davis, E.J.; Saydah, S.; Imperatore, G.; Linder, B.; Divers, J.; Bell, R.; Badaru, A.; Talton, J.W.; Crume, T.; et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. J. Am. Med. Assoc. (JAMA) 2014, 311, 1778–1786. [Google Scholar] [CrossRef]
- DIAMOND Project Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990–1999. Diabet. Med. 2006, 23, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Van Belle, T.L.; Coppieters, K.T.; von Herrath, M.G. Type 1 diabetes: Etiology, immunology, and therapeutic strategies. Physiol. Rev. 2011, 91, 79–118. [Google Scholar] [CrossRef]
- Oresic, M. Metabolomics in the studies of islet autoimmunity and type 1 diabetes. Rev. Diabet. Stud. 2012, 9, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, A.J.; Kaur, S.; Pociot, F. Metabolomic biomarkers in the progression to type 1 diabetes. Curr. Diabetes Rep. 2016, 16, 127. [Google Scholar] [CrossRef] [PubMed]
- Gooding, J.R.; Jensen, M.V.; Newgard, C.B. Metabolomics applied to the pancreatic islet. Arch. Biochem. Biophys. 2016, 589, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Frohnert, B.I.; Rewers, M.J. Metabolomics in childhood diabetes. Pediatric Diabetes 2016, 17, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Delovitch, T.L.; Singh, B. The nonobese diabetic mouse as a model of autoimmune diabetes: Immune dysregulation gets the NOD. Immunity 1997, 7, 727–738. [Google Scholar] [CrossRef]
- Leiter, E.H. The NOD mouse: A model for insulin-dependent diabetes mellitus. Curr. Protoc. Immunol. 2001, 24, 15.19.11–15.19.23. [Google Scholar]
- Anderson, M.S.; Bluestone, J.A. The NOD mouse: A model of immune dysregulation. Annu. Rev. Immunol. 2005, 23, 447–485. [Google Scholar] [CrossRef]
- Roep, B.O.; Atkinson, M.; von Herrath, M. Satisfaction (not) guaranteed: Re-evaluating the use of animal models of type 1 diabetes. Nat. Rev. Immunol. 2004, 4, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Shoda, L.K.; Young, D.L.; Ramanujan, S.; Whiting, C.C.; Atkinson, M.A.; Bluestone, J.A.; Eisenbarth, G.S.; Mathis, D.; Rossini, A.A.; Campbell, S.E.; et al. A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity 2005, 23, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Leiter, E.H.; Schile, A. Genetic and pharmacologic models for type 1 diabetes. Curr. Protoc. Mouse Biol. 2013, 3, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.C.; Herold, K.C. Thinking bedside at the bench: The NOD mouse model of T1DM. Nat. Rev. Endocrinol. 2015, 11, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Abdulreda, M.H.; Berman, D.M.; Shishido, A.; Martin, C.; Hossameldin, M.; Tschiggfrie, A.; Hernandez, L.F.; Hernandez, A.; Ricordi, C.; Parel, J.M.; et al. Operational immune tolerance towards transplanted allogeneic pancreatic islets in mice and a non-human primate. Diabetologia 2019, 62, 811–821. [Google Scholar] [CrossRef] [Green Version]
- Abdulreda, M.H.; Molano, R.D.; Faleo, G.; Lopez-Cabezas, M.; Shishido, A.; Ulissi, U.; Fotino, C.; Hernandez, L.H.; Tschiggfrie, A.; Aldrich, V.R.; et al. In vivo imaging of type 1 diabetes immunopathology using eye-transplanted islets in NOD mice. Diabetologia 2019, 62, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- Bachmayr-Heyda, A.; Aust, S.; Auer, K.; Meier, S.M.; Schmetterer, K.G.; Dekan, S.; Gerner, C.; Pils, D. Integrative systemic and local metabolomics with impact on survival in high-grade serous ovarian cancer. Clin. Cancer Res. 2017, 23, 2081–2092. [Google Scholar] [CrossRef] [PubMed]
- Schoeman, J.C.; Harms, A.C.; van Weeghel, M.; Berger, R.; Vreeken, R.J.; Hankemeier, T. Development and application of a UHPLC-MS/MS metabolomics based comprehensive systemic and tissue-specific screening method for inflammatory, oxidative and nitrosative stress. Anal. Bioanal. Chem. 2018, 410, 2551–2568. [Google Scholar] [CrossRef]
- Duncan, K.D.; Fyrestam, J.; Lanekoff, I. Advances in mass spectrometry based single-cell metabolomics. Analyst 2019, 144, 782–793. [Google Scholar] [CrossRef]
- Buchwald, P.; Tamayo-Garcia, A.; Ramamoorthy, S.; Garcia-Contreras, M.; Mendez, A.J.; Ricordi, C. A comprehensive metabolomics study to assess longitudinal biochemical changes and potential early biomarkers in NOD mice that progress to diabetes. J. Proteome Res. 2017, 16, 3873–3890. [Google Scholar] [CrossRef] [PubMed]
- Owei, I.; Umekwe, N.; Stentz, F.; Wan, J.; Dagogo-Jack, S. Amino acid signature predictive of incident prediabetes: A case-control study nested within the longitudinal pathobiology of prediabetes in a biracial cohort. Metabolism 2019, 98, 76–83. [Google Scholar] [CrossRef]
- Bender, K.; Newsholme, P.; Brennan, L.; Maechler, P. The importance of redox shuttles to pancreatic beta-cell energy metabolism and function. Biochem. Soc. Trans. 2006, 34, 811–814. [Google Scholar] [CrossRef]
- Newsholme, P.; Brennan, L.; Rubi, B.; Maechler, P. New insights into amino acid metabolism, beta-cell function and diabetes. Clin. Sci. 2005, 108, 185–194. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef]
- Cechin, S.R.; Lopez-Ocejo, O.; Ricordi, C.; Buchwald, P. Smad7 is a promising therapeutic target on the TGF-β pathway to prevent or reverse new-onset type 1 diabetes. Diabetes 2015, 64, A60. [Google Scholar]
- Cechin, S.R.; Lopez-Ocejo, O.; Karpinsky-Semper, D.; Buchwald, P. Biphasic decline of -cell function with age in euglycemic nonobese diabetic mice parallels diabetes onset. IUBMB Life 2015, 67, 634–644. [Google Scholar] [CrossRef]
- Abdulreda, M.H.; Caicedo, A.; Berggren, P.-O. Transplantation into the anterior chamber of the eye for longitudinal, non-invasive in vivo imaging with single-cell resolution in real-time. J. Vis. Exp. 2013, 73, e50466. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, L.; Rada, P.; Paredes, D.; Hernandez, L. In vivo monitoring of cerebral agmatine by microdialysis and capillary electrophoresis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 880, 58–65. [Google Scholar] [CrossRef]
- Betancourt, L.; Rada, P.; Hernandez, L.; Araujo, H.; Ceballos, G.A.; Hernandez, L.E.; Tucci, P.; Mari, Z.; De Pasquale, M.; Paredes, D.A. Micellar electrokinetic chromatography with laser induced fluorescence detection shows increase of putrescine in erythrocytes of Parkinson’s disease patients. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1081–1082, 51–57. [Google Scholar] [CrossRef]
- Abdulreda, M.H.; Faleo, G.; Molano, R.D.; Lopez-Cabezas, M.; Molina, J.; Tan, Y.; Echeverria, O.A.; Zahr-Akrawi, E.; Rodriguez-Diaz, R.; Edlund, P.K.; et al. High-resolution, noninvasive longitudinal live imaging of immune responses. Proc. Natl. Acad. Sci. USA 2011, 108, 12863–12868. [Google Scholar] [CrossRef] [Green Version]
- Miska, J.; Abdulreda, M.H.; Devarajan, P.; Lui, J.B.; Suzuki, J.; Pileggi, A.; Berggren, P.O.; Chen, Z. Real-time immune cell interactions in target tissue during autoimmune-induced damage and graft tolerance. J. Exp. Med. 2014, 211, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Dungan, K.M. 1,5-anhydroglucitol (GlycoMark) as a marker of short-term glycemic control and glycemic excursions. Expert Rev. Mol. Diagn. 2008, 8, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Taboulet, P.; Deconinck, N.; Thurel, A.; Haas, L.; Manamani, J.; Porcher, R.; Schmit, C.; Fontaine, J.P.; Gautier, J.F. Correlation between urine ketones (acetoacetate) and capillary blood ketones (3-beta-hydroxybutyrate) in hyperglycaemic patients. Diabetes Metab. 2007, 33, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Klocker, A.A.; Phelan, H.; Twigg, S.M.; Craig, M.E. Blood beta-hydroxybutyrate vs. urine acetoacetate testing for the prevention and management of ketoacidosis in Type 1 diabetes: A systematic review. Diabet. Med. 2013, 30, 818–824. [Google Scholar] [CrossRef]
- Christen, U.; Wolfe, T.; Mohrle, U.; Hughes, A.C.; Rodrigo, E.; Green, E.A.; Flavell, R.A.; von Herrath, M.G. A dual role for TNF-alpha in type 1 diabetes: Islet-specific expression abrogates the ongoing autoimmune process when induced late but not early during pathogenesis. J. Immunol. 2001, 166, 7023–7032. [Google Scholar] [CrossRef]
- Krause, M.S.; McClenaghan, N.H.; Flatt, P.R.; de Bittencourt, P.I.; Murphy, C.; Newsholme, P. L-arginine is essential for pancreatic β-cell functional integrity, metabolism and defense from inflammatory challenge. J. Endocrinol. 2011, 211, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef]
- Garcia-Contreras, M.; Tamayo-Garcia, A.; Pappan, K.L.; Michelotti, G.A.; Stabler, C.L.; Ricordi, C.; Buchwald, P. A metabolomics study of the effects of inflammation, hypoxia, and high glucose on isolated human pancreatic islets. J. Proteome Res. 2017, 16, 2294–2306. [Google Scholar] [CrossRef] [PubMed]
- Murfitt, S.A.; Zaccone, P.; Wang, X.; Acharjee, A.; Sawyer, Y.; Koulman, A.; Roberts, L.D.; Cooke, A.; Griffin, J. A metabolomics and lipidomics study of mouse models of type 1 diabetes highlights divergent metabolism in purine and tryptophan metabolism prior to disease on-set. J. Proteome Res. 2017, 17, 946–960. [Google Scholar] [CrossRef]
- Frumento, G.; Rotondo, R.; Tonetti, M.; Damonte, G.; Benatti, U.; Ferrara, G.B. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J. Exp. Med. 2002, 196, 459–468. [Google Scholar] [CrossRef]
- Moffett, J.R.; Namboodiri, M.A. Tryptophan and the immune response. Immunol. Cell Biol. 2003, 81, 247–265. [Google Scholar] [CrossRef]
- Munn, D.H.; Mellor, A.L. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013, 34, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, 369. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Raynal, S.; Bailbe, D.; Gausseres, B.; Carbonne, C.; Autier, V.; Movassat, J.; Kergoat, M.; Portha, B. Expression of the kynurenine pathway enzymes in the pancreatic islet cells. Activation by cytokines and glucolipotoxicity. Biochim. Biophys. Acta 2015, 1852, 980–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, M.H. The targeting of immunosuppressive mechanisms in hematological malignancies. Leukemia 2014, 28, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Curran, T.A.; Jalili, R.B.; Farrokhi, A.; Ghahary, A. IDO expressing fibroblasts promote the expansion of antigen specific regulatory T cells. Immunobiology 2014, 219, 17–24. [Google Scholar] [CrossRef]
- Grohmann, U.; Orabona, C.; Fallarino, F.; Vacca, C.; Calcinaro, F.; Falorni, A.; Candeloro, P.; Belladonna, M.L.; Bianchi, R.; Fioretti, M.C.; et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat. Immunol. 2002, 3, 1097–1101. [Google Scholar] [CrossRef]
- Sakiani, S.; Olsen, N.J.; Kovacs, W.J. Gonadal steroids and humoral immunity. Nat. Rev. Endocrinol. 2013, 9, 56–62. [Google Scholar] [CrossRef]
- Zandman-Goddard, G.; Peeva, E.; Shoenfeld, Y. Gender and autoimmunity. Autoimmun. Rev. 2007, 6, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Bultman, S.J.; Holley, D.; Hillhouse, C.; Bain, J.R.; Newgard, C.B.; Muehlbauer, M.J.; Willis, M.S. Non-targeted metabolomics of Brg1/Brm double-mutant cardiomyocytes reveals a novel role for SWI/SNF complexes in metabolic homeostasis. Metabolomics 2015, 11, 1287–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kind, T.; Wohlgemuth, G.; Lee, D.Y.; Lu, Y.; Palazoglu, M.; Shahbaz, S.; Fiehn, O. FiehnLib: Mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem. 2009, 81, 10038–10048. [Google Scholar] [CrossRef] [PubMed]
- Styczynski, M.P.; Moxley, J.F.; Tong, L.V.; Walther, J.L.; Jensen, K.L.; Stephanopoulos, G.N. Systematic identification of conserved metabolites in GC/MS data for metabolomics and biomarker discovery. Anal. Chem. 2007, 79, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcazar, O.; Hernandez, L.F.; Tschiggfrie, A.; Muehlbauer, M.J.; Bain, J.R.; Buchwald, P.; Abdulreda, M.H. Feasibility of Localized Metabolomics in the Study of Pancreatic Islets and Diabetes. Metabolites 2019, 9, 207. https://doi.org/10.3390/metabo9100207
Alcazar O, Hernandez LF, Tschiggfrie A, Muehlbauer MJ, Bain JR, Buchwald P, Abdulreda MH. Feasibility of Localized Metabolomics in the Study of Pancreatic Islets and Diabetes. Metabolites. 2019; 9(10):207. https://doi.org/10.3390/metabo9100207
Chicago/Turabian StyleAlcazar, Oscar, Luis F. Hernandez, Ashley Tschiggfrie, Michael J. Muehlbauer, James R. Bain, Peter Buchwald, and Midhat H. Abdulreda. 2019. "Feasibility of Localized Metabolomics in the Study of Pancreatic Islets and Diabetes" Metabolites 9, no. 10: 207. https://doi.org/10.3390/metabo9100207
APA StyleAlcazar, O., Hernandez, L. F., Tschiggfrie, A., Muehlbauer, M. J., Bain, J. R., Buchwald, P., & Abdulreda, M. H. (2019). Feasibility of Localized Metabolomics in the Study of Pancreatic Islets and Diabetes. Metabolites, 9(10), 207. https://doi.org/10.3390/metabo9100207