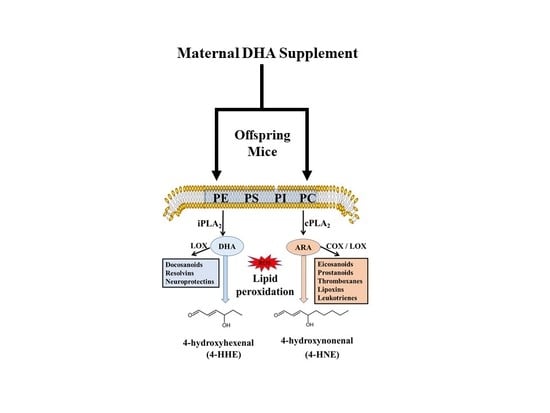
Maternal Dietary Docosahexaenoic Acid Alters Lipid Peroxidation Products and (n-3)/(n-6) Fatty Acid Balance in Offspring Mice
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, G.Y.; Simonyi, A.; Fritsche, K.L.; Chuang, D.Y.; Hannink, M.; Gu, Z.; Greenlief, C.M.; Yao, J.K.; Lee, J.C.; Beversdorf, D.Q. Docosahexaenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Yavin, E.; Glozman, S.; Green, P. Docosahexaenoic acid sources for the developing brain during intrauterine life. Nutr. Health 2001, 15, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, J. Docosahexaenoic acid (DHA): An ancient nutrient for the modern human brain. Nutrients 2011, 3, 529–554. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Zhang, M.; Wang, L.; Li, H.; Cai, H.; Dang, R.; Jiang, P.; Liu, Y.; Xue, Y.; Wu, Y. Maternal dietary of n-3 polyunsaturated fatty acids affects the neurogenesis and neurochemical in female rat at weaning. Prostaglandins Leukot. Essent. Fat. Acids 2018, 128, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Lyall, K.; Munger, K.L.; O’Reilly, E.J.; Santangelo, S.L.; Ascherio, A. Maternal dietary fat intake in association with autism spectrum disorders. Am. J. Epidemiol. 2013, 178, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Ooi, Y.P.; Weng, S.J.; Kossowsky, J.; Gerger, H.; Sung, M. Oxytocin and Autism Spectrum Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmacopsychiatry 2017, 50, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Keim, S.A.; Gracious, B.; Boone, K.M.; Klebanoff, M.A.; Rogers, L.K.; Rausch, J.; Coury, D.L.; Sheppard, K.W.; Husk, J.; Rhoda, D.A. omega-3 and omega-6 Fatty Acid Supplementation May Reduce Autism Symptoms Based on Parent Report in Preterm Toddlers. J. Nutr. 2018, 148, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Julvez, J.; Mendez, M.; Fernandez-Barres, S.; Romaguera, D.; Vioque, J.; Llop, S.; Ibarluzea, J.; Guxens, M.; Avella-Garcia, C.; Tardon, A.; et al. Maternal Consumption of Seafood in Pregnancy and Child Neuropsychological Development: A Longitudinal Study Based on a Population With High Consumption Levels. Am. J. Epidemiol. 2016, 183, 169–182. [Google Scholar] [CrossRef] [PubMed]
- James, S.; Montgomery, P.; Williams, K. Omega-3 fatty acids supplementation for autism spectrum disorders (ASD). Cochrane Database Syst. Rev. 2011, CD007992. [Google Scholar] [CrossRef] [PubMed]
- Horvath, A.; Lukasik, J.; Szajewska, H. omega-3 Fatty Acid Supplementation Does Not Affect Autism Spectrum Disorder in Children: A Systematic Review and Meta-Analysis. J. Nutr. 2017, 147, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Mankad, D.; Dupuis, A.; Smile, S.; Roberts, W.; Brian, J.; Lui, T.; Genore, L.; Zaghloul, D.; Iaboni, A.; Marcon, P.M.; et al. A randomized, placebo controlled trial of omega-3 fatty acids in the treatment of young children with autism. Mol. Autism 2015, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Zhang, M.; Cai, H.; Li, H.; Jiang, P.; Dang, R.; Liu, Y.; He, X.; Xue, Y.; Cao, L.; et al. Maternal diet of polyunsaturated fatty acid altered the cell proliferation in the dentate gyrus of hippocampus and influenced glutamatergic and serotoninergic systems of neonatal female rats. Lipids Health Dis. 2016, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Matsui, F.; Hecht, P.; Yoshimoto, K.; Watanabe, Y.; Morimoto, M.; Fritsche, K.; Will, M.; Beversdorf, D. DHA Mitigates Autistic Behaviors Accompanied by Dopaminergic Change in a Gene/Prenatal Stress Mouse Model. Neuroscience 2017, 371, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Kevala, K.; Kim, J.; Moon, H.S.; Jun, S.B.; Lovinger, D.; Kim, H.Y. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J. Neurochem. 2009, 111, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Asatryan, A.; Bazan, N.G. Molecular mechanisms of signaling via the docosanoid neuroprotectin D1 for cellular homeostasis and neuroprotection. J. Biol. Chem. 2017, 292, 12390–12397. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiu, V.; Leuti, A.; Dalli, J.; Jacobsson, A.; Battistini, L.; Maccarrone, M.; Serhan, C.N. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci. Transl. Med. 2016, 8, 353ra111. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.L.; Smith, R.M.; Edwards, K.S.; Givens, B.; Tilley, M.R.; Beversdorf, D.Q. Combined effect of maternal serotonin transporter genotype and prenatal stress in modulating offspring social interaction in mice. Int. J. Dev. Neurosci. 2010, 28, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.L.; Will, M.J.; Hecht, P.M.; Parker, C.L.; Beversdorf, D.Q. Maternal diet rich in omega-6 polyunsaturated fatty acids during gestation and lactation produces autistic-like sociability deficits in adult offspring. Behav. Brain Res. 2013, 238, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.J.; Mucha, B.; Denheyer, H.; Atkinson, D.; Schanz, N.; Vassiliou, E.; Benno, R.H. Dietary docosahexaenoic acid alleviates autistic-like behaviors resulting from maternal immune activation in mice. Prostaglandins Leukot. Essent. Fat. Acids 2016, 106, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Guichardant, M.; Bacot, S.; Moliere, P.; Lagarde, M. Hydroxy-alkenals from the peroxidation of n-3 and n-6 fatty acids and urinary metabolites. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Wong, H.Y.; Chai, Y.Y.; Shi, C.W.; Amino, N.; Kikuchi, S.; Huang, S.H. Lipid peroxidation dysregulation in ischemic stroke: Plasma 4-HNE as a potential biomarker? Biochem. Biophys. Res. Commun. 2012, 425, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, S.; Mao, L.; Leak, R.K.; Shi, Y.; Zhang, W.; Hu, X.; Sun, B.; Cao, G.; Gao, Y.; et al. Omega-3 fatty acids protect the brain against ischemic injury by activating Nrf2 and upregulating heme oxygenase 1. J. Neurosci. 2014, 34, 1903–1915. [Google Scholar] [CrossRef] [PubMed]
- Moniuszko-Malinowska, A.; Luczaj, W.; Jarocka-Karpowicz, I.; Pancewicz, S.; Zajkowska, J.; Andrisic, L.; Zarkovic, N.; Skrzydlewska, E. Lipid peroxidation in the pathogenesis of neuroborreliosis. Free Radic. Biol. Med. 2016, 96, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Luczaj, W.; Moniuszko, A.; Jarocka-Karpowicz, I.; Pancewicz, S.; Andrisic, L.; Zarkovic, N.; Skrzydlewska, E. Tick-borne encephalitis--lipid peroxidation and its consequences. Scand. J. Clin. Lab. Investig. 2016, 76, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Luczaj, W.; Gegotek, A.; Skrzydlewska, E. Antioxidants and HNE in redox homeostasis. Free Radic. Biol. Med. 2017, 111, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Csala, M.; Kardon, T.; Legeza, B.; Lizak, B.; Mandl, J.; Margittai, E.; Puskas, F.; Szaraz, P.; Szelenyi, P.; Banhegyi, G. On the role of 4-hydroxynonenal in health and disease. Biochim. Biophys. Acta 2015, 1852, 826–838. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wang, M.; Han, X. Shotgun lipidomics in substantiating lipid peroxidation in redox biology: Methods and applications. Redox Biol. 2017, 12, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Long, E.K.; Picklo, M.J., Sr. Trans-4-hydroxy-2-hexenal, a product of n-3 fatty acid peroxidation: Make some room HNE. Free Radic. Biol. Med. 2010, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, F.; Morino, K.; Ugi, S.; Ishikado, A.; Kondo, K.; Sato, D.; Konno, S.; Nemoto, K.; Kusunoki, C.; Sekine, O.; et al. 4-Hydroxyhexenal derived from dietary n-3 polyunsaturated fatty acids induces anti-oxidative enzyme heme oxygenase-1 in multiple organs. Biochem. Biophys. Res. Commun. 2014, 443, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.Y.; Horrocks, L.A. The fatty acid and aldehyde composition of the major phospholipids of mouse brain. Lipids 1968, 3, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Calderon, F.; Kim, H.Y. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J. Neurochem. 2004, 90, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Li, R.; Greenlief, C.M.; Fritsche, K.L.; Gu, Z.; Cui, J.; Lee, J.C.; Beversdorf, D.Q.; Sun, G.Y. Unveiling anti-oxidative and anti-inflammatory effects of docosahexaenoic acid and its lipid peroxidation product on lipopolysaccharide-stimulated BV-2 microglial cells. J. Neuroinflamm. 2018, 15, 202. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Fauconnier, J.; Oger, C.; Farah, C.; Angebault-Prouteau, C.; Thireau, J.; Bideaux, P.; Scheuermann, V.; Bultel-Ponce, V.; Demion, M.; et al. Non-enzymatic oxidized metabolite of DHA, 4(RS)-4-F4t-neuroprostane protects the heart against reperfusion injury. Free Radic. Biol. Med. 2017, 102, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G. Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Chawla, A.; Loayza, M.S.; Bazan, N.G. Docosanoids are multifunctional regulators of neural cell integrity and fate: Significance in aging and disease. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Novel chemical mediators in the resolution of inflammation: Resolvins and protectins. Anesthesiol. Clin. 2006, 24, 341–364. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.H.; Paris, C.; Magnien, M.; Colin, J.; Pelleieux, S.; Coste, F.; Escanye, M.C.; Pillot, T.; Olivier, J.L. Dietary arachidonic acid increases deleterious effects of amyloid-beta oligomers on learning abilities and expression of AMPA receptors: Putative role of the ACSL4-cPLA2 balance. Alzheimer’s Res. Ther. 2017, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Hopperton, K.E.; Trepanier, M.O.; James, N.C.E.; Chouinard-Watkins, R.; Bazinet, R.P. Fish oil feeding attenuates neuroinflammatory gene expression without concomitant changes in brain eicosanoids and docosanoids in a mouse model of Alzheimer’s disease. Brain Behav. Immun. 2018, 69, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Hopperton, K.E.; Trepanier, M.O.; Giuliano, V.; Bazinet, R.P. Brain omega-3 polyunsaturated fatty acids modulate microglia cell number and morphology in response to intracerebroventricular amyloid-beta 1-40 in mice. J. Neuroinflamm. 2016, 13, 257. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Yoshida, Y.; Saito, Y.; Noguchi, N.; Niki, E. Adaptive response induced by lipid peroxidation products in cell cultures. FEBS Lett. 2006, 580, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Riahi, Y.; Sunda, V.; Deplano, S.; Chatgilialoglu, C.; Ferreri, C.; Kaiser, N.; Sasson, S. Signaling properties of 4-hydroxyalkenals formed by lipid peroxidation in diabetes. Free Radic. Biol. Med. 2013, 65, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Domenichiello, A.F.; Kitson, A.P.; Bazinet, R.P. Is docosahexaenoic acid synthesis from alpha-linolenic acid sufficient to supply the adult brain? Prog. Lipid Res. 2015, 59, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Lepage, G.; Roy, C.C. Improved recovery of fatty acid through direct transesterification without prior extraction or purification. J. Lipid Res. 1984, 25, 1391–1396. [Google Scholar] [PubMed]
- Johnson, M.C.; Song, H.; Cui, J.; Mossine, V.V.; Gu, Z.; Greenlief, C.M. Development of a Method and Validation for the Quantitation of FruArg in Mice Plasma and Brain Tissue Using UPLC-MS/MS. ACS Omega 2016, 1, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.Y.; Li, R.; Yang, B.; Fritsche, K.L.; Beversdorf, D.Q.; Lubahn, D.B.; Geng, X.; Lee, J.C.; Greenlief, C.M. Quercetin Potentiates Docosahexaenoic Acid to Suppress Lipopolysaccharide-induced Oxidative/Inflammatory Responses, Alter Lipid Peroxidation Products, and Enhance the Adaptive Stress Pathways in BV-2 Microglial Cells. Int. J. Mol. Sci. 2019, 20, 932. [Google Scholar] [CrossRef] [PubMed]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Li, R.; Woo, T.; Browning, J.D., Jr.; Song, H.; Gu, Z.; Cui, J.; Lee, J.C.; Fritsche, K.L.; Beversdorf, D.Q.; et al. Maternal Dietary Docosahexaenoic Acid Alters Lipid Peroxidation Products and (n-3)/(n-6) Fatty Acid Balance in Offspring Mice. Metabolites 2019, 9, 40. https://doi.org/10.3390/metabo9030040
Yang B, Li R, Woo T, Browning JD Jr., Song H, Gu Z, Cui J, Lee JC, Fritsche KL, Beversdorf DQ, et al. Maternal Dietary Docosahexaenoic Acid Alters Lipid Peroxidation Products and (n-3)/(n-6) Fatty Acid Balance in Offspring Mice. Metabolites. 2019; 9(3):40. https://doi.org/10.3390/metabo9030040
Chicago/Turabian StyleYang, Bo, Runting Li, Taeseon Woo, Jimmy D. Browning, Jr., Hailong Song, Zezong Gu, Jiankun Cui, James C. Lee, Kevin L. Fritsche, David Q. Beversdorf, and et al. 2019. "Maternal Dietary Docosahexaenoic Acid Alters Lipid Peroxidation Products and (n-3)/(n-6) Fatty Acid Balance in Offspring Mice" Metabolites 9, no. 3: 40. https://doi.org/10.3390/metabo9030040
APA StyleYang, B., Li, R., Woo, T., Browning, J. D., Jr., Song, H., Gu, Z., Cui, J., Lee, J. C., Fritsche, K. L., Beversdorf, D. Q., Sun, G. Y., & Greenlief, C. M. (2019). Maternal Dietary Docosahexaenoic Acid Alters Lipid Peroxidation Products and (n-3)/(n-6) Fatty Acid Balance in Offspring Mice. Metabolites, 9(3), 40. https://doi.org/10.3390/metabo9030040