Blueberry-Based Meals for Obese Patients with Metabolic Syndrome: A Multidisciplinary Metabolomic Pilot Study
Abstract
:1. Introduction
2. Results and Discussion
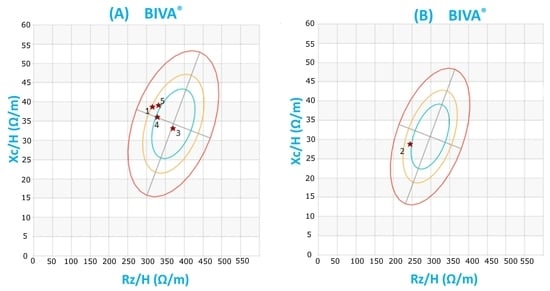
2.1. Anthropometric Characteristics
2.2. Blueberry Meals
2.3. NMR Data and Statistical Analysis
2.3.1. V60—Ketone Bodies
2.3.2. V57—Succinate and Tricarboxylic Acid (TCA) Cycle
2.3.3. V49 and V47—Dimethylamine (DMA) and Trimethylamine (TMA)
2.3.4. V25 and V21—p-Hydroxyphenyl-Acetic (HPA) and 3-(3’-Hydroxyphenyl)-3-Hydropropionic (HPHPA) Acids
2.4. Pro- and Anti-Inflammatory Cytokines: Real-Time Quantitative PCR Analysis
3. Materials and Methods
3.1. Experimental Design
3.2. Body Composition Analysis (BCA)
3.3. Bioelectrical Impedance Analysis (BIA)
3.4. NMR Data Acquisition
3.5. Real-Time Quantitative PCR Analysis
3.6. Ethical Statement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, L.; Sun, Z.; Zeng, Y.; Luo, M.; Yang, J. Molecular mechanism and health role of functional ingredients in blueberry for chronic disease in human beings. Int. J. Mol. Sci. 2018, 19, 2785. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardener, H.; Wright, C.B.; Gu, Y.; Demmer, R.T.; Boden-Albala, B.; Elkind, M.; Sacco, R.L.; Scarmeas, N. Mediterranean-style diet and risk of ischemic stroke, myocardial infarction, and vascular death: The Northern Manhattan Study. Am. J. Clin. Nutr. 2011, 94, 1458–1464. [Google Scholar] [CrossRef]
- Muraki, I.; Imamura, F.; Manson, J.E.; Hu, F.B.; Willett, W.C.; Van Dam, R.M.; Sun, Q. Fruit consumption and risk of type 2 diabetes: Results from three prospective longitudinal cohort studies. BMJ 2013, 347, f5001. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Rochette, N.; Da, S.V.M.; Nabavi, S.M.; Mota, E.F.; Nunes-Pinheiro, D.C.; Daglia, M.; De, M.D.F. Fruit as potent natural antioxidants and their biological effects. Curr. Pharm. Biotechnol. 2016, 17, 986–993. [Google Scholar] [CrossRef]
- McAnulty, L.S.; Nieman, D.C.; Dumke, C.L.; Shooter, L.A.; Henson, D.A.; Utter, A.C.; Milne, G.; McAnulty, S.R. Effect of blueberry ingestion on natural killer cell counts, oxidative stress, and inflammation prior to and after 2.5 h of running. Appl. Physiol. Nutr. Metab. 2011, 36, 976–984. [Google Scholar] [CrossRef]
- Lee, Y.M.; Yoon, Y.; Yoon, H.; Park, H.M.; Song, S.; Yeum, K.J. Dietary anthocyanins against obesity and inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef]
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48. [Google Scholar] [CrossRef]
- Barton, R.H.; Nicholson, J.K.; Elliott, P.; Holmes, E. High-throughput 1H NMR-based metabolic analysis of human serum and urine for large-scale epidemiological studies: Validation study. Int. J. Epidemiol. 2008, 37, i31–i40. [Google Scholar] [CrossRef]
- Keun, H.C.; Ebbels, T.M.; Antti, H.; Bollard, M.E.; Beckonert, O.; Schlotterbeck, G.; Senn, H.; Niederhauser, U.; Holmes, E.; Lindon, J.C. Analytical reproducibility in 1H NMR-based metabonomic urinalysis. Chem. Res. Toxicol. 2002, 15, 1380–1386. [Google Scholar] [CrossRef]
- Percival, B.C.; Grootveld, M.; Gibson, M.; Osman, Y.; Molinari, M.; Jafari, F.; Sahota, T.; Martin, M.; Casanova, F.; Mather, M.L.; et al. Low-Field, Benchtop NMR Spectroscopy as a Potential Tool for Point-of-Care Diagnostics of Metabolic Conditions: Validation, Protocols and Computational Models. High Throughput 2018, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Businaro, R.; Corsi, M.; Asprino, R.; Di Lorenzo, C.; Laskin, D.; Corbo, R.M.; Ricci, S.; Pinto, A. Modulation of Inflammation as a Way of Delaying Alzheimer’s Disease Progression: The Diet’s Role. Curr. Alzheimer Res. 2018, 15, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Blacker, B.C.; Snyder, S.M.; Eggett, D.L.; Parker, T.L. Consumption of blueberries with a high-carbohydrate, low-fat breakfast decreases postprandial serum markers of oxidation. Br. J. Nutr. 2013, 109, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Edirisinghe, I.; Banaszewski, K.; Cappozzo, J.; Sandhya, K.; Ellis, C.L.; Tadapaneni, R.; Kappagoda, C.T.; Burton-Freeman, B.M. Strawberry anthocyanin and its association with postprandial inflammation and insulin. Br. J. Nutr. 2011, 106, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Special Communication. JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, A.; Rossi, B.; Pillon, L.; Bucciante, G. A new method for monitoring body fluid variation by bioimpedance analysis: The RXc graph. Kidney Int. 1994, 46, 534–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coin, A.; Sergi, G.; Minicuci, N.; Giannini, S.; Barbiero, E.; Manzato, E.; Pedrazzoni, M.; Minisola, S.; Rossini, M.; Del Puente, A.; et al. Fat-free mass and fat mass reference values by dual-energy X-ray absorptiometry (DEXA) in a 20-80 year-old Italian population. Clin. Nutr. 2008, 27, 87–94. [Google Scholar] [CrossRef]
- Xiao, J.; Purcell, S.A.; Prado, C.M.; Gonzalez, M.C. Fat mass to fat-free mass ratio reference values from NHANES III using bioelectrical impedance analysis. Clin. Nutr. 2018, 37, 2284–2287. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Poggiogalle, E.; Lubrano, C.; Sergi, G.; Coin, A.; Gnessi, L.; Mariani, S.; Lenzi, A.; Donini, L.M. Sarcopenic Obesity and Metabolic Syndrome in Adult Caucasian Subjects. J. Nutr. Health Aging 2016, 20, 958–963. [Google Scholar] [CrossRef]
- Emerson, S.R.; Kurti, S.P.; Harms, C.A.; Haub, M.D.; Melgarejo, T.; Logan, C.; Rosenkranz, S.K. Magnitude and Timing of the Postprandial Inflammatory Response to a High-Fat Meal in Healthy Adults: A Systematic Review. Adv. Nutr. 2017, 8, 213–225. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Bernini, P.; Bertini, I.; Luchinat, C.; Nincheri, P.; Staderini, S.; Turano, P. Standard operating procedures for pre-analytical handling of blood and urine for metabolomics studies and biobank. J. Biomol. NMR 2011, 49, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Vignoli, A.; Rodio, D.M.; Bellizzi, A.; Sobolev, A.P.; Anzivino, E.; Mischitelli, M.; Tenori, L.; Marini, F.; Priori, R.; Scrivo, R.; et al. NMR-based metabolomic approach to study urine samples of chronic inflammatory rheumatic disease patients. Anal. Bioanal. Chem. 2017, 409, 1405–1413. [Google Scholar] [CrossRef]
- Messana, I.; Forni, F.; Ferrari, F.; Rossi, C.; Giardina, B.; Zuppi, C. Proton nuclear magnetic resonance spectral profiles of urine in type II diabetic patients. Clin. Chem. 1998, 44, 1529–1534. [Google Scholar] [PubMed]
- Liu, H.; Tayyari, F.; Edison, A.S.; Zhihua, S.; Gu, L. NMR-based metabonomics reveals urinary metabolome modifications in female Sprague-Dawley rats by cranberry procyanidins. J. Nutr. Biochem. 2016, 34, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Huang, D. Dietary Antioxidants and Health Promotion. Antioxidants 2018, 7, 9. [Google Scholar] [CrossRef]
- Nicholson, J.K.; O’flynn, M.P.; Salder, P.J. Proton-nuclear-magnetic-resonance studies of serum, plasma and urine from fasting normal and diabetic subjects. Biochem. J. 1984, 217, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.M.; Kuhara, T.; Inoue, Y.; Matsumoto, I.; Iwasaki, R.; Morimoto, S. Increased urinary excretion of beta-hydroxyisovaleric acid in ketotic and non-ketotic type II diabetes mellitus. Clin. Chim. Acta 1990, 188, 161–168. [Google Scholar] [CrossRef]
- Laffel, L. Ketone bodies: A review of Physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab. Res. Rev. 1999, 15, 412–426. [Google Scholar] [CrossRef]
- Salek, R.M.; Maguire, M.L.; Bentley, E.; Rubtsov, D.V.; Hough, T.; Cheeseman, M.; Nunez, D.; Sweatman, B.C.; Haselden, J.N.; Cox, R.D.; et al. A metabonomic comparison of urinary changes in type 2 diabetes in mouse, rat, and human. Physiol. Genom. 2007, 29, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Xie, L.; Diao, C.; Wang, N.; Hu, W.; Zheng, Y.; Jin, L.; Yan, Z.; Gao, H. Systemic Perturbations of Key Metabolites in Diabetic Rats During the Evolution of Diabetes Studied by Urine Metabonomics. PLoS ONE 2013, 8, e60409. [Google Scholar] [CrossRef] [PubMed]
- Millis, E.; O’neill, L.A. Succinate: A metabolic signal in inflammation. Trends Cell Biol. 2014, 24, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Perez, A.; Burg, M.B. Role of organic osmolytes in adaptation of renal cells to high osmolality. J. Membr. Biol. 1991, 119, 1–13. [Google Scholar] [CrossRef] [PubMed]
- AI-Obaide, M.A.I.; Singh, R.; Datta, P.; Rewers-Felkins, K.A.; Salguero, M.V.; AI-Obaidi, I.; Kottapalli, K.R.; Vasylyeva, T.L. Gut microbiota-dependent Trimethylamine-N-oxide and serum biomarkers in patients with T2DM and advanced CKD. J. Clin. Med. 2017, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Stoupi, S.; Williamson, G.; Drynan, J.W.; Barron, D.; Clifford, M.N. A comparison of the in vitro biotrasformation of (-) -epicatechin and procyanidin B2 by human faecal microbiota. Mol. Nutr. Food Res. 2010, 54, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Wedick, N.M.; Pan, A.; Cassidy, A.; Rimm, E.B.; Sampson, L.; Rosner, B.; Willett, W.; Hu, F.B.; Sun, Q.; van Dam, R.M. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am. J. Clin. Nutr. 2012, 95, 925–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, F.C.J.A. Inhibitory effects of blueberry extract on the production of inflammatory mediators in lipopolysaccharide-activated BV2 microglia. J. Neurosci. Res. 2007, 85, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Yan, H.; Han, C.; Wang, W.; Tian, Y.; Chen, X. Polyphenols from blueberries modulate inflammation cytokines in LPS-induced RAW264.7 macrophages. Int. J. Biol. Macromol. 2014, 69, 382–387. [Google Scholar] [CrossRef]
- Xing, Z.; Jordana, M.; Kirpalani, H.; Driscoll, K.E.; Schall, T.J.; Gauldie, J. Cytokine expression by neutrophils and macrophages in vivo: Endotoxin induces tumor necrosis factor -α, macrophage inflammatory protein-2, interleukin-1 beta, and interleukin-6 but not RANTES or transforming growth factor-β 1 mRNA expression in acute lung inflammation. Am. J. Respir. Cell Mol. Biol. 1994, 10, 148–153. [Google Scholar] [PubMed]
- Hobbs, S.; Reynoso, M.; Geddis, A.V.; Mitrophanov, A.Y.; Matheny, R.W., Jr. LPS-stimulated NF-κB p65 dynamic response marks the initiation of TNF expression and transition to IL-10 expression in RAW 264.7 macrophages. Physiol. Rep. 2018, 6, e13914. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.N.; Fisher, D.R.; Rimando, A.M.; Gomes, S.M.; Bielinski, D.F.; Shukitt-Hale, B. Stilbenes and anthocyanins reduce stress signaling in BV-2 mouse microglia. J. Agric. Food Chem. 2013, 61, 5979–5986. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Xu, M.; Ren, M.; Pan, E.; Luo, C.; Zhang, W.; Tang, Q. Immunomodulatory Effect of Flavonoids of Blueberry (Vaccinium corymbosum L.) Leaves via the NF-κB Signal Pathway in LPS-Stimulated RAW 264.7 Cells. J. Immunol. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Gao, Y.; Guo, X.; Zhang, M.; Gong, L. Blackberry and Blueberry Anthocyanin Supplementation Counteract High-Fat-Diet-Induced Obesity by Alleviating Oxidative Stress and Inflammation and Accelerating Energy Expenditure. Oxid. Med. Cell. Longev. 2018. [Google Scholar] [CrossRef]
- Gildawie, K.R.; Rovnak, A.; Thangthaeng, N.; Fisher, D.R.; Shukitt-Hale, B. Blueberry supplementation attenuates microglia activation and increases neuroplasticity in mice consuming a high-fat diet. Nutr. Neurosci. 2017, 21, 1–11. [Google Scholar]
- Xie, C.; Kang, J.; Ferguson, M.E.; Nagarajan, S.; Badger, T.M.; Wu, X. Blueberries reduce pro-inflammatory cytokine TNF-α and IL-6 production in mouse macrophages by inhibiting NF-κB activation and the MAPK pathway. Mol. Nutr. Food Res. 2011, 55, 1587–1591. [Google Scholar] [CrossRef]
- DeFuria, J.; Bennett, G.; Strissel, K.J.; Perfield, J.W.; Milbury, P.E.; Greenberg, A.S.; Obin, M.S. Dietary blueberry attenuates whole-body insulin resistance in high fat-fed mice by reducing adipocyte death and its inflammatory sequelae. J. Nutr. 2009, 139, 1510–1516. [Google Scholar] [CrossRef]
- Shukitt-Hale, B.; Lau, F.C.; Carey, A.N.; Galli, R.L.; Spangler, E.L.; Ingram, D.K.; Joseph, J.A. Blueberry polyphenols attenuate kainic acid-induced decrements in cognition and alter inflammatory gene expression in rat hippocampus. Nutr. Neurosci. 2008, 11, 172–182. [Google Scholar] [CrossRef]
- Figueira, M.E.; Oliveira, M.; Direito, R.; Rocha, J.; Alves, P.; Serra, A.T.; Duarte, C.; Bronze, R.; Fernandes, A.; Brites, D.; et al. Protective effects of a blueberry extract in acute inflammation and collagen-induced arthritis in the rat. Biomed. Pharmacother. 2016, 83, 1191–1202. [Google Scholar] [CrossRef]
- Zhong, S.; Sandhu, A.; Edirisinghe, I.; Burton-Freeman, B. Characterization of Wild Blueberry Polyphenols Bioavailability and Kinetic Profile in Plasma over 24-h Period in Human Subjects. Mol. Nutr. Food Res. 2017, 61, 12. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Feresin, R.G.; Navaei, N.; Figueroa, A.; Elam, M.L.; Akhavan, N.S.; Hooshmand, S.; Pourafshar, S.; Payton, M.E.; Arjmandi, B.H. Effects of daily blueberry consumption on circulating biomarkers of oxidative stress, inflammation, and antioxidant defense in postmenopausal women with pre- and stage 1-hypertension: A randomized controlled trial. Food Funct. 2017, 8, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Thakali, K.M.; Jensen, G.; Wu, X. Phenolic Acids of the Two Major Blueberry Species in the US Market and Their Antioxidant and Anti-inflammatory Activities. Plant Foods Hum. Nutr. 2015, 70, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Regulska-Ilow, B. The significance of anthocyanins in the prevention and treatment of type 2 diabetes. Adv. Clin. Exp. Med. 2018, 27, 135–142. [Google Scholar] [Green Version]
- Gowd, V.; Jia, Z.; Chen, W. Anthocyanins as promising molecules and dietary bioactive components against diabetes–A review of recent advances. Trends Food Sci. Technol. 2017, 68, 1–13. [Google Scholar] [CrossRef]
- Castro-Acosta, M.L.; Lenihan-Geels, G.N.; Corpe, C.P.; Hall, W.L. Berries and anthocyanins: Promising functional food ingredients with postprandial glycaemia-lowering effects. Proc. Nutr. Soc. 2016, 75, 342–355. [Google Scholar] [CrossRef]
- Kowalska, K.; Olejnik, A. Current evidence on the health-beneficial effects of berry fruits in the prevention and treatment of metabolic syndrome. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 446–452. [Google Scholar] [CrossRef]
- Basu, A.; Du, M.; Leyva, M.J.; Sanchez, K.; Betts, N.M.; Wu, M.; Aston, C.E.; Lyons, T.J. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J. Nutr. 2010, 140, 1582–1587. [Google Scholar] [CrossRef]
- Stull, A.J.; Cash, K.C.; Johnson, W.D.; Champagne, C.M.; Cefalu, W.T. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J. Nutr. 2010, 140, 1764–1768. [Google Scholar] [CrossRef]
- Vendrame, S.; Del Bo, C.; Ciappellano, S.; Riso, P.; Klimis-Zacas, D. Berry Fruit Consumption and Metabolic Syndrome. Antioxidants 2016, 5, 34. [Google Scholar] [CrossRef]
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Berries: Anti-inflammatory effects in humans. J. Agric. Food Chem. 2014, 62, 3886–3903. [Google Scholar] [CrossRef] [PubMed]
- Naseri, R.; Farzaei, F.; Haratipour, P.; Nabavi, S.F.; Habtemariam, S.; Farzaei, M.H.; Khodarahmi, R.; Tewari, D.; Momtaz, S. Anthocyanins in the Management of Metabolic Syndrome: A Pharmacological and Biopharmaceutical Review. Front. Pharmacol. 2018, 9, 1310. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.R.; Mariappan, N.; Stull, A.J.; Francis, J. Blueberry supplementation attenuates oxidative stress within monocytes and modulates immune cell levels in adults with metabolic syndrome: A randomized, double-blind placebo-controlled trial. Food Funct. 2017, 8, 4118–4128. [Google Scholar] [CrossRef]
- Wu, X.; Wang, T.T.Y.; Prior, R.L.; Pehrsson, P.R. Prevention of Atherosclerosis by Berries: The Case of Blueberries. J. Agric. Food Chem. 2018, 66, 9172–9188. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, S.; Gerring, E.; Gjellan, S.; Vergara, M.; Lindström, T.; Nystrom, F.H. Effects of exercise with or without blueberries in the diet on cardio-metabolic risk factors: An exploratory pilot study in healthy subjects. Ups. J. Med. Sci. 2013, 118, 247–255. [Google Scholar] [CrossRef]
- Gordon, C.C.; Chumlea, W.C.; Roche, A.F. Statura, lunghezza supina e peso. In Manuale di Riferimento per la Standardizzazione Antropometrica, 1st ed.; Battistini, N.C., Bedogni, G.M., Eds.; Edra: Milano, Italy, 1992; Chapter 1; pp. 5–20. [Google Scholar]
- Callaway, C.W.; Chumlea, W.C.; Bouchard, C.; Himes, H.H.; Lohman, T.G.; Martin, A.D.; Mitchell, C.D.; Mueller, W.H.; Roche, A.F.; Seefeldt, V.D. Circonferenze. In Manuale di Riferimento per la Standardizzazione Antropometrica, 1st ed.; Battistini, N.C., Bedogni, G.M., Eds.; Edra: Milano, Italy, 1992; Chapter 5; pp. 51–65. [Google Scholar]
- Kushner, R.F. Bioelectrical impedance analysis: A review of principles and applications. J. Am. Coll. Nutr. 1992, 11, 199–209. [Google Scholar]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 1985, 89, 465–471. [Google Scholar] [CrossRef]
- Kim, J.; Heshka, S.; Gallagher, D.; Kotler, D.P.; Mayer, L.; Albu, J.; Shen, W.; Freda, P.U.; Heymsfield, S.B. Intermuscular adipose tissue-free skeletal muscle mass: Estimation by dual-energy X-ray absorptiometry in adults. J. Appl. Physiol. 1985, 97, 655–660. [Google Scholar] [CrossRef]
- Human Metabolome Database. Available online: http://www.hmdb.ca (accessed on 1 October 2018).
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic Quotient Normalization as Robust Method to Account for Dilution of Complex Biological Mixtures. Application in 1H NMR Metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]





| Subject. | Subject 1 | Subject 2 | Subject 3 | Subject 4 | Subject 5 |
|---|---|---|---|---|---|
| Gender | F | M | F | F | F |
| Age (y) | 52 | 52 | 61 | 25 | 31 |
| Height (cm) | 156 | 174.5 | 158 | 170 | 152 |
| Weight (kg) | 87.1 | 90.1 | 71.7 | 84.9 | 92.8 |
| BMI (kg/m2) | 35.78 | 29.58 | 28.71 | 29.37 | 40.17 |
| WC (cm) | 103.0 | 100.5 | 92.5 | 91.0 | 115.0 |
| Z50kHz (Ω) | 497 | 431 | 589 | 564.8 | 509.8 |
| PhA (°) | 7.0 | 6.7 | 5.1 | 6.2 | 6.7 |
| Rz50kHz (Ω) | 493.3 | 428.1 | 586.7 | 561.5 | 506.3 |
| Xc50kHz (Ω) | 60.6 | 43.7 | 52.4 | 61.4 | 59.6 |
| FM (kg) | 36.9 | 21.6 | 27.6 | 33.6 | 44.2 |
| FM % | 42.4 | 24.0 | 38.5 | 39.5 | 47.6 |
| FFM (kg) | 50.2 | 68.5 | 44.1 | 51.3 | 48.6 |
| FFM % | 57.6 | 76 | 61.5 | 60.5 | 52.4 |
| FFMI (kg/m2) | 20.6 | 22.5 | 17.7 | 17.8 | 21 |
| FMI (kg/m2) | 15.2 | 7.1 | 11.1 | 11.6 | 19.1 |
| FM (kg)/FFM (kg) | 0.74 | 0.32 | 0.63 | 0.65 | 0.91 |
| TBW (L) | 36.6 | 50.3 | 32.4 | 37.6 | 35.4 |
| ECW (L) | 15.2 | 21.5 | 16.3 | 16.8 | 15.1 |
| ICW (L) | 21.6 | 28.8 | 16.1 | 20.8 | 20.3 |
| BCM (kg) | 29.3 | 39.1 | 21.6 | 28.2 | 27.8 |
| SM (kg) | 23.3 | 33.8 | 17.8 | 23.9 | 21.2 |
| SMI (kg/m2) | 9.6 | 11.1 | 7.1 | 8.3 | 9.2 |
| ASMM (kg) | 20.5 | 27.5 | 15.9 | 20.8 | 19.9 |
| ASMM/height2 (kg/m2) | 8.42 | 9.03 | 6.37 | 7.20 | 8.61 |
| ASMM/weight | 0.2354 | 0.3052 | 0.2218 | 0.2450 | 0.2144 |
| “A” Meal | Protein (g) | Carbohydrates (g) | Lipids (g) | Calories (Kcal) | |
| Potatoes | 400 g | 8.4 | 71.6 | 4.0 | 338.1 |
| Bread | 50 g | 4.5 | 28.8 | 0.95 | 134.6 |
| Baked Ham | 60 g | 9.4 | 1.0 | 4.6 | 82.6 |
| “Mozzarella” Cheese | 60 g | 11.2 | 0.4 | 11.7 | 151.8 |
| Butter | 20 g | 0.16 | 0.22 | 16.7 | 151.6 |
| “Parmigiano” Cheese | 10 g | 3.35 | 0 | 2.8 | 38.7 |
| Blueberries | 150 g | 1.35 | 7.65 | 0.3 | 36.8 |
| Total | 38.4(16.4%) | 109.7(44%) | 41(39.5%) | 934.01 | |
| CHOs (13.6 g) | SFA (21.6 g) | GL (70) | |||
| CHO (86.4 g) | MUFA (3.8 g) | ||||
| PUFA (11.2 g) | |||||
| “B” Meal | Protein (g) | Carbohydrates (g) | Lipids (g) | Calories (Kcal) | |
| Potatoes | 400 g | 8.4 | 71.6 | 4.0 | 338.1 |
| Bread | 60 g | 5.4 | 34.6 | 1.14 | 161.5 |
| Baked Ham | 60 g | 9.4 | 1.0 | 4.6 | 82.6 |
| “Mozzarella” Cheese | 60 g | 11.2 | 0.4 | 11.7 | 151.8 |
| Butter | 20 g | 0.16 | 0.22 | 16.7 | 151.6 |
| “Parmigiano” Cheese | 10 g | 3.35 | 0 | 2.8 | 38.7 |
| Total | 37.95(16.4%) | 107.8(43.8%) | 41(40.89%) | 924.14 | |
| CHOs (6.7 g) | SFA (21.6 g) | GL (70) | |||
| CHO (90.9 g) | MUFA (3.6 g) | ||||
| PUFA (11.2 g) |
| Variable/(Spectral Range) | Assignment | F | p-Level |
|---|---|---|---|
| V60 (2.17–2.21) ppm | Acetoacetate/Acetone | 16.5 | 0.00091 |
| V57 (2.39–2.40) ppm | Succinate | 6.3 | 0.02332 |
| V49 (2.72–2.74) ppm | Dimethylamine (DMA) | 6.0 | 0.02614 |
| V47 (2.91–2.94) ppm | Trimethylamine (TMA) | 6.2 | 0.02383 |
| V25 (6.85–6.89) ppm | 3-(3’-Hydroxyphenyl)-3-hydropropionic acid/ p-Hydroxyphenyl-acetic acid | 4.7 | 0.04517 |
| V21 (7.14–7.22) ppm | 3-(3’-Hydroxyphenyl)-3-hydropropionic acid/ p-Hydroxyphenyl-acetic acid/histidine | 7.5 | 0.01442 |
| Cytokines | p-Level |
|---|---|
| IL-1β | 0.807 |
| IL-6 | 0.0014 |
| TNF-α | 0.5824 |
| IL-10 | 0.9807 |
| IL-4 | 0.0809 |
| TGF-β | 0.0038 |
| Gene | Forward Primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| hIL1 β | GCTTATTACAGTGGCAATGAGG | GGTGGTCGGAGATTCGTAG |
| hIL6 | GGTACATCCTCGACGGCATCT | GTGCCTCTTTGCTGCTTTCAC |
| hTNFα | ATCTTCTCGAACCCCGAGTGA | CGGTTCAGCCACTGGAGCT |
| hIL4 | ACTGCACAGCAGTTCCACAG | CTCTGGTTGGCTTCCTTCAC |
| hIL10 | GATGCCTTCAGCAGAGTGAA | GCAACCCAGGTAACCCTTAAA |
| hTGF β | GCAGAGCTGCGTCTGCTGAGGC | CCCGTTGATGTCCACTTGCAGTG |
| hGAPDH | ACAGTCAGCCGCATCTTC | GCCCAATACGACCAAATCC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobolev, A.P.; Ciampa, A.; Ingallina, C.; Mannina, L.; Capitani, D.; Ernesti, I.; Maggi, E.; Businaro, R.; Del Ben, M.; Engel, P.; et al. Blueberry-Based Meals for Obese Patients with Metabolic Syndrome: A Multidisciplinary Metabolomic Pilot Study. Metabolites 2019, 9, 138. https://doi.org/10.3390/metabo9070138
Sobolev AP, Ciampa A, Ingallina C, Mannina L, Capitani D, Ernesti I, Maggi E, Businaro R, Del Ben M, Engel P, et al. Blueberry-Based Meals for Obese Patients with Metabolic Syndrome: A Multidisciplinary Metabolomic Pilot Study. Metabolites. 2019; 9(7):138. https://doi.org/10.3390/metabo9070138
Chicago/Turabian StyleSobolev, Anatoly Petrovich, Alessandra Ciampa, Cinzia Ingallina, Luisa Mannina, Donatella Capitani, Ilaria Ernesti, Elisa Maggi, Rita Businaro, Maria Del Ben, Petra Engel, and et al. 2019. "Blueberry-Based Meals for Obese Patients with Metabolic Syndrome: A Multidisciplinary Metabolomic Pilot Study" Metabolites 9, no. 7: 138. https://doi.org/10.3390/metabo9070138
APA StyleSobolev, A. P., Ciampa, A., Ingallina, C., Mannina, L., Capitani, D., Ernesti, I., Maggi, E., Businaro, R., Del Ben, M., Engel, P., Giusti, A. M., Donini, L. M., & Pinto, A. (2019). Blueberry-Based Meals for Obese Patients with Metabolic Syndrome: A Multidisciplinary Metabolomic Pilot Study. Metabolites, 9(7), 138. https://doi.org/10.3390/metabo9070138