Neuroprotective γ-Pyrones from Fusarium Solani JS-0169: Cell-Based Identification of Active Compounds and an Informatics Approach to Predict the Mechanism of Action
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Fungal Materials
2.3. Extraction and Isolations
6-((9R,11R,E)-13-hydroxy-9,11-dimethyloct-7-en-7-yl)-2-methoxy-4H-pyran-4-one (1)
2.4. 1-diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging Activity
2.5. Cell Culture and Treatment.
2.6. Measurement of Cell Viability
2.7. Systems Pharmacological Analysis
2.8. Molecular Docking
3. Results
3.1. Identification of Compounds 1–6
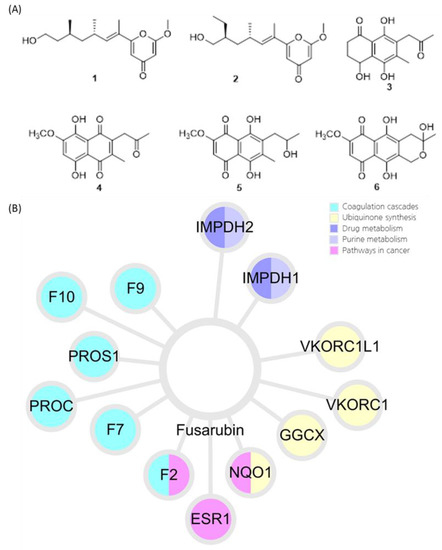
3.2. Identification of Active Compound and Informatics Approach to Predict the Mechanism of Action
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Murphy, T.H.; Miyamoto, M.; Sastre, A.; Schnaar, R.L.; Coyle, J.T. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron 1989, 2, 1547–1558. [Google Scholar] [CrossRef]
- Coyle, J.T.; Puttfarcken, P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993, 262, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Ishige, K.; Schubert, D.; Sagara, Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic. Biol. Med. 2001, 30, 433–446. [Google Scholar] [CrossRef]
- Harvey, A.L.; Cree, I.A. High-throughput screening of natural products for cancer therapy. Planta Med. 2010, 76, 1080–1086. [Google Scholar] [CrossRef] [Green Version]
- Rahman, I.; Chung, S. Dietary polyphenols, deacetylases and chromatin remodeling in inflammation. J. Nutr. Nutr. 2010, 3, 220–230. [Google Scholar]
- Bagachi, A.; Semwal, A.; Bharadwaj, A. Traditional uses, phytochemistry and pharmacology of Morus alba Linn.: A review. J. Med. Plant Res. 2013, 7, 461–469. [Google Scholar]
- Zhang, Y.; Du, W.; Zhang, X.; Zhao, H.; Wang, Y. Antioxidant activity and the potential for cholesterol-lowering of phenolic extract of Morus alba, Morus multicaulis, and Morus laevigata leaves from Yunnan (China). J. Food Biochem. 2017, 41, e12339. [Google Scholar] [CrossRef]
- Du, J.; He, Z.D.; Jiang, R.W.; Ye, W.C.; Xu, H.X.; But, P.P.H. Antiviral flavonoids from the root bark of Morus alba L. Phytochemistry 2003, 62, 1235–1238. [Google Scholar] [CrossRef]
- Kim, D.S.; Kang, Y.M.; Jin, W.Y.; Sung, Y.Y.; Choi, G.; Kim, H.K. Antioxidant activities and polyphenol content of Morus alba leaf extracts collected from varying regions. Biomed. Rep. 2014, 2, 675–680. [Google Scholar] [CrossRef] [Green Version]
- Nomura, T.; Fukai, T. Hypotensive constituent, kuwanon-H, a new flavone derivative from the root bark of the cultivated mulberry tree (Morus alba L.). Heterocycles 1980, 14, 1943–1951. [Google Scholar] [CrossRef]
- Seo, K.H.; Lee, D.Y.; Jeong, R.H.; Lee, D.S.; Kim, Y.E.; Hong, E.K.; Kim, Y.C.; Baek, N.I. Neuroprotective effect of prenylated arylbenzofuran and flavonoids from Morus alba fruits on glutamate-induced oxidative injury in HT22 hippocampal cells. J. Med. Food 2015, 18, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Garyali, S.; Kumar, A.; Reddy, M.S. Taxol production by an endophytic fungus, Fusarium redolens, isolated from Himalayan yew. J. Microbiol. Biotechnol. 2013, 23, 1372–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nirmaladevi, D.; Venkataramana, M.; Chandranayaka, S.; Ramesha, A.; Jameel, N.M.; Srinivas, C. Neuroprotective effects of bikaverin on H2O2-induced oxidative stress mediated neuronal damage in SH-SY5Y cell line. Cell. Mol. Neurobiol. 2014, 34, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Barabasi, A.L.; Gulbahce, N.; Loscalzo, J. Network medicine: A network-based approach to human disease. Nat. Rev. Genet. 2011, 12, 56–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Wu, M.; Wang, H.; Li, S.; Wang, X.; Li, Y.; Wang, D.; Li, S. Network Pharmacology to Unveil the Biological Basis of Health-Strengthening Herbal Medicine in Cancer Treatment. Cancers 2018, 10, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.Y.; Park, J.H.; Kim, H.S.; Lee, C.Y.; Lee, H.J.; Kang, K.S.; Kim, C.E. Systems-level mechanisms of action of Panax ginseng: A network pharmacological approach. J. Ginseng Res. 2018, 42, 98–106. [Google Scholar] [CrossRef]
- Park, M.; Park, S.Y.; Lee, H.J.; Kim, C.E. A Systems-Level Analysis of Mechanisms of Platycodon grandiflorum Based on A Network Pharmacological Approach. Molecules 2018, 23, 2841. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Guo, F.; Wang, Y.; Li, C.; Zhang, X.; Li, H.; Diao, L.; Gu, J.; Wang, W.; Li, D.; et al. BATMAN-TCM: A Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine. Sci. Rep. 2016, 6, 21146. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [Green Version]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef] [Green Version]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.N.; Wang, Z.C.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.; Skalic, M.; Martinez-Rosell, G.; De Fabritiis, G. KDEEP: Protein-Ligand Absolute Binding Affinity Prediction via 3D-Convolutional Neural Networks. J. Chem. Inf. Model. 2018, 58, 287–296. [Google Scholar] [CrossRef]
- Gong, J.; Chen, C.; Mo, S.; Liu, J.; Wang, W.; Zang, Y.; Li, H.; Chai, C.; Zhu, H.; Hu, Z.; et al. Fusaresters A–E, new γ-pyrone-containing polyketides from fungus Fusarium sp. Hungcl and structure revision of fusariumin D. Org. Biomol. Chem. 2019, 17, 5526–5532. [Google Scholar] [CrossRef]
- Khan, N.; Afroz, F.; Begum, M.N.; Rony, S.R.; Sharmin, S.; Moni, F.; Mahmood Hasan, C.; Shaha, K.; Sohrab, M.H. Endophytic Fusarium solani: A rich source of cytotoxic and antimicrobial napthaquinone and aza-anthraquinone derivatives. Toxicol. Rep. 2018, 5, 970–976. [Google Scholar] [CrossRef]
- Kumar, K.P.; Javvaji, K.; Poornachandra, Y. Antimicrobial, anti-plasmodial and cytotoxicity properties of bioactive compounds from Fusarium sp. USNPF102. J. Microbiol. Res. 2017, 7, 23–30. [Google Scholar]
- Hashimoto, J.; Motohashi, K.; Sakamoto, K.; Hashimoto, S.; Yamanouchi, M.; Tanaka, H.; Takahashi, T.; Takagi, M.; Shin-Ya, K. Screening and evaluation of new inhibitors of hepatic glucose production. J. Antibiot. 2009, 62, 625–629. [Google Scholar] [CrossRef]
- Takemoto, K.; Kamisuki, S.; Chia, P.T.; Kuriyama, I.; Mizushina, Y.; Sugawara, F. Bioactive dihydronaphthoquinone derivatives from Fusarium solani. J. Nat. Prod. 2014, 77, 1992–1996. [Google Scholar] [CrossRef]
- Siemieniuk, E.; Skrzydlewska, E. Coenzyme Q10: Its biosynthesis and biological significance in animal organisms and in humans. Postepy Hig. Med. Dosw. 2005, 59, 150–159. [Google Scholar]
- Littarru, G.P.; Tiano, L. Bioenergetic and antioxidant properties of coenzyme Q10: Recent developments. Mol. Biotechnol. 2007, 37, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Bironaite, D.A.; Cenas, N.K.; Anusevicius, Z.J.; Medentsev, A.G.; Akimenko, V.K.; Usanov, S.A. Fungal quinone pigments as oxidizers and inhibitors of mitochondrial NADH:ubiquinone reductase. Arch. Biochem. Biophys. 1992, 297, 253–257. [Google Scholar] [CrossRef]
- Ross, D.; Kepa, J.K.; Winski, S.L.; Beall, H.D.; Anwar, A.; Siegel, D. NAD(P)H:quinone oxidoreductase 1 (NQO1): Chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem. Biol. Interact. 2000, 129, 77–97. [Google Scholar] [CrossRef]
- Harvey, A.L.; Clark, R.L.; Mackay, S.P.; Johnston, B.F. Current strategies for drug discovery through natural products. Expert Opin. Drug Discov. 2010, 5, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Hwang, O. Role of oxidative stress in Parkinson’s disease. Exp. Neurobiol. 2013, 22, 461–491. [Google Scholar] [CrossRef] [Green Version]
- Bartke, N.; Hannun, Y.A. Bioactive sphingolipids: Metabolism and function. J. Lipid Res. 2009, 50, S91–S96. [Google Scholar] [CrossRef] [Green Version]
- Ferland, G. Vitamin K and the nervous system: An overview of its actions. Adv. Nutr. 2012, 3, 204–212. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Chen, J.; Hammonds, G.; Phillips, H.; Armanini, M.; Wood, P.; Bunge, R.; Godowski, P.J.; Sliwkowski, M.X.; Mather, J.P. Identification of Gas6 as a growth factor for human Schwann cells. J. Neurosci. 1996, 16, 2012–2019. [Google Scholar] [CrossRef] [Green Version]







| Ligand Name | Molecular Weight | pKd | ΔG (kcal/mol) |
|---|---|---|---|
| Fusarubin | 306.07 | 7.8 | −10.53 |
| ARH | 322.13 | 7.55 | −10.19 |
| FAD | 785.16 | 6.8 | −9.18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.G.; Song, J.H.; Park, M.; Kim, S.; Kim, C.-E.; Kang, K.S.; Shim, S.H. Neuroprotective γ-Pyrones from Fusarium Solani JS-0169: Cell-Based Identification of Active Compounds and an Informatics Approach to Predict the Mechanism of Action. Biomolecules 2020, 10, 91. https://doi.org/10.3390/biom10010091
Choi HG, Song JH, Park M, Kim S, Kim C-E, Kang KS, Shim SH. Neuroprotective γ-Pyrones from Fusarium Solani JS-0169: Cell-Based Identification of Active Compounds and an Informatics Approach to Predict the Mechanism of Action. Biomolecules. 2020; 10(1):91. https://doi.org/10.3390/biom10010091
Chicago/Turabian StyleChoi, Hyun Gyu, Ji Hoon Song, Musun Park, Soonok Kim, Chang-Eop Kim, Ki Sung Kang, and Sang Hee Shim. 2020. "Neuroprotective γ-Pyrones from Fusarium Solani JS-0169: Cell-Based Identification of Active Compounds and an Informatics Approach to Predict the Mechanism of Action" Biomolecules 10, no. 1: 91. https://doi.org/10.3390/biom10010091
APA StyleChoi, H. G., Song, J. H., Park, M., Kim, S., Kim, C. -E., Kang, K. S., & Shim, S. H. (2020). Neuroprotective γ-Pyrones from Fusarium Solani JS-0169: Cell-Based Identification of Active Compounds and an Informatics Approach to Predict the Mechanism of Action. Biomolecules, 10(1), 91. https://doi.org/10.3390/biom10010091