1. Introduction
Hashimoto’s thyroiditis (HT), an autoimmune thyroid disease (AITD), is characterized by infiltration of thyroid antigen-reactive T cells and by the presence of autoantibodies against thyroid antigens (thyroid peroxidase, TPO; thyroglobulin, Tg). Thyroid autoimmunity results in thyrocyte lysis and destruction of thyroid tissue, leading to hypothyroidism [
1,
2].
Studies of in vitro models have shown that the mechanisms of thyroid tissue damage in AITD rely on antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) triggered by autoantibodies characteristic for AITD. The role of autoantibodies against thyroid antigens in thyrocyte-destroying ADCC and CDC processes was demonstrated using in vitro models at the end of the last century. Thyroid cell lysis was significantly more intensive in the presence of HT sera than with healthy-subject sera in ADCC assays performed in vitro using peripheral blood lymphocytes from healthy donors as the effector cells. Lysis of thyroid target cells was also more severe when they were exposed in the absence of serum to effector cells isolated from the blood of HT patients, as compared with thyrocyte lysis in the presence of effector cells from healthy donors [
3]. Rodient and coworkers showed that anti-TPO from thyroiditis sera, but not anti-Tg, participates in ADCC of porcine thyrocytes in primary culture. Thyrocyte lysis triggered by anti-TPO- and anti-Tg-depleted IgG was significantly decreased in comparison to cell death in the presence of the total serum IgG pool [
4]. A study of an in vitro model consisting of primary human thyroid cells used as the target cells, serum samples from HT, Graves’ disease (GD) and primary myxoedema donors used as autoantibody sources, and PBMC serving as the effector cells confirmed the significance of thyroid-specific antibodies in the ADCC process. Thyrocyte lysis was significantly higher for each of the three patient groups versus control sera, and HT-specific antibodies were more effective than GD autoantibodies in triggering thyroid cell lysis [
5]. The involvement of anti-TPO in CDC has also been shown in vitro. HT IgGs exerted the cytotoxic effect of the complement more efficiently than control antibodies did. Pre-incubation of HT IgG with purified TPO significantly decreased specific cell lysis, demonstrating that CDC is mediated by anti-TPO IgG. This effect was not observed in CDC with the use of HT IgG pre-incubated with purified Tg [
6]. Rebuffat and coworkers confirmed the importance of anti-TPO in thyroid destruction, also using in vitro models of ADCC and CDC. Thyroid primary cells lysed in a process initiated by anti-TPO and effected by PBMC or HL-60 and THP-1 human monocyte cell lines. Highly effective lysis of thyrocytes by CDC was also observed when the cells were incubated with anti-TPO followed by guinea pig serum used as complement source [
7].
IgG mediates an effector function via the ability to interact with its Fcγ receptor (FcγR) expressed on effector cells and with a C1q complement protein. It is well established that
N-glycans attached to IgG Fc greatly contribute to the regulation of IgG effector functions [
8]. IgG could induce pro- or anti-inflammatory signals, depending on the sugar composition of Fc
N-glycans. Low core-fucosylation of Fc
N-glycan enhances ADCC [
9]. In general, terminal sialylation is responsible for anti-inflammatory activity, but the final effect of IgG sialylation depends on the monosaccharide composition of Fc
N-glycans [
10]. Modifications of Fc
N-glycan structures are commonly used in therapeutic antibodies to modulate the efficiency of Fc binding to its receptor and to increase the effectiveness of therapy [
11]. Changes of IgG
N-glycosylation have been noted in different chronic diseases, including cancers [
12,
13] and inflammatory disorders [
14,
15,
16,
17]. Although analysis of IgG glycosylation has become a standard approach in work aimed at discovering new plasma biomarkers, functional analysis of altered IgG glycosylation remains largely neglected. Previously we demonstrated the altered
N-glycosylation profile of IgG from Hashimoto’s thyroiditis patients. We observed significantly lower IgG core fucosylation in HT than in healthy individuals [
18]. Analysis of IgG-depleted sera from HT donors indicated an increase of α2,3-sialylated di- and tri-antennary complex-type
N-glycans on serum proteins [
19].
Based on these recent studies, we hypothesized that altered
N-glycosylation of serum proteins affects pathological processes occurring in HT, including thyroid destruction in ADCC and CDC processes. The present study is a functional analysis of differentially
N-glycosylated IgG isolated from HT patients and healthy donors (control group) in in vitro models of ADCC and CDC. Normal and cancerous thyroid cell lines were used as target cells in both cytotoxicity models. Peripheral blood mononuclear cells (PBMCs) and the HL-60 cell line served as the effector cells in the ADCC model, and normal serum as an effector component in the CDC assay. We used cytotoxic assays (
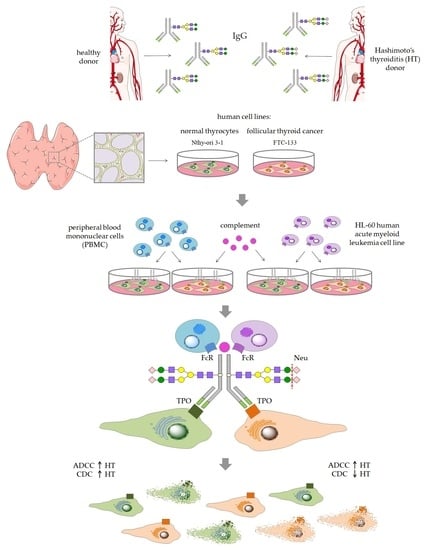
Figure 1) in a comparative analysis of thyroid cell lysis triggered by control and HT IgG, both enzymatically desialylated.
2. Materials and Methods
2.1. Ethical Statement
The study was conducted in accordance with the Helsinki Declaration and the protocol was approved by the Bioethics Committee of Jagiellonian University (Permit No. 1072.6120.145.2017). All blood donors gave their informed consent for inclusion before their participation in the study.
2.2. IgG Isolation
Blood samples were collected from HT female patients of the Endocrinology Clinic of the University Hospital in Kraków and from age- and sex-matched healthy individuals (control group). All donors were recruited to the study based on serum levels of TSH, anti-TPO and anti-Tg, and on thyroid ultrasonography. Blood was collected via venous puncture into clotting activator tubes (S-Monovette, Sarstedt), left for 5 h at RT for blood coagulation, and centrifuged (2500 rpm, 10 min, RT). Serum samples were kept stored under deep freezing until IgG isolation or CDC assay. IgG was isolated using protein G affinity chromatography. Serum samples (100 µL) from healthy donors (n = 24) and HT patients (n = 24) were diluted 1:1 with phosphate buffer (0.1 M sodium phosphate, 0.15 M sodium chloride, pH 7.5) and applied to a Protein G Spin Plate (Thermo Scientific, Waltham, MA USA; 45204). After repeated washing with the phosphate buffer, IgG was eluted with 0.1 M glycine (pH 2.5) and immediately neutralized with 1 M Tris (pH 9). IgG samples purified from the sera of 24 donors were pooled for further procedures.
2.3. Desialylation of IgG
Before desialylation, IgG eluted in glycine buffer was subjected to buffer exchange for PBS (Sigma-Aldrich, St. Louis, MO, USA; P4417), using Amicon Ultra 10 kDa filters (Millipore, Burlington, MA, USA). Then, α2-3,6,8-neuraminidase (Neu) from
Clostridium perfringens (New England BioLabs, Ipswich, MA, USA; P0720) was used to remove sialic acid (SA) from IgG
N-glycans. IgG (100 µg) was incubated with 200 U Neu in 40 µL of 50 mM sodium acetate containing 5 mM CaCl
2 (pH 5.5) and incubated overnight at 37 °C. After desialylation, Neu was removed using VivaSpin filters (Sartorius, Göttingen, Germany), with a molecular weight cut-off of 50 kDa. The efficiency of desialylation was monitored using
Sambucus nigra agglutinin (SNA) in lectin blotting (see
Section 2.8).
2.4. Cell Lines and Culture Condition
The Nthy-ori 3-1 human thyroid follicular epithelial cell line and FTC-133 human follicular thyroid cancer cell line were kindly provided by Prof. Barbara Czarnocka of the Centre of Postgraduate Medical Education in Warsaw and by Prof. Anna Krześlak of the University of Łódź, respectively. Human acute myeloid leukemia HL-60 cell line was obtained from Dr. Małgorzata Opydo-Chanek of the Jagiellonian University. The cells used to the experiments were mycoplasma-free, as routinely determined by a chemiluminescence test (Lonza, Basel, Switzerland) and verified by PCR (forward primer: 5′-ACTCCTACGGGAGGCAGCAGTA-3′, reverse primer: 5′-TGCACCATCTGTCACTCTGTTAACCTC-3′, Oligo). The use of the Nthy-ori 3-1 cell line was reported to the Ministry of the Environment, because Nthy-ori 3-1 was qualified as a genetically modified microorganism (GMM).
Nthy-ori 3-1 and HL-60 were maintained in RPMI 1640 medium (Lonza, Basel, Switzerland; BE12-702F) supplemented with 10% FBS (Gibco, Paisley, UK; 10270-106) and antibiotics (100 units/mL of penicillin and 100 μg/mL of streptomycin, Sigma-Aldrich, P4333). FTC-133 was cultured in DMEM (Sigma-Aldrich, St. Louis, MO, USA; D5671) supplemented with 10% FBS and antibiotics. The cells were kept in a humidified incubator with 95% air and 5% CO2 at 37 °C. After confluence, Nthy-ori 3-1 and FTC-133 cells were subdivided into new flasks when ~80% confluence was reached. HL-60 cells were passaged after reaching cell density of 25 × 104 cells/mL.
2.5. RT-qPCR
TPO mRNA expression in thyroid cell lines was determined by isolation of total cellular RNA using an RNeasy Plus Mini Kit (Qiagen, Hilden, Germany) and reverse-transcribed into cDNA using a High-Capacity RNA-to-cDNA Kit (Applied Biosystems, Foster City, CA, USA). cDNA was subjected to real-time quantitative reverse transcription PCR (RT-qPCR) using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA). The following primers were used for the TPO gene: 5′-TTGTACAACGGGTTCCCACT-3′ (forward) and 5′-GGAGGTCAGAATAGCGGTCA-3′ (reverse); and for the 18S rRNA reference gene: 5′-CCAGTAAGTGCGGGTCATAAG-3′ (forward) and 5′-CCATCCAATCGGTAGTAGCG-3′ (reverse). RT-qPCR data were quantified by the 2−ΔΔCt method. The experiment was performed in triplicate.
2.6. SDS-PAGE and Immunodetection of TPO
Total cell extracts were obtained using RIPA buffer (Thermo Fisher Scientific, Waltham, MA USA) containing a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA; P8340). Equal amounts (25 µg) of Nthy-ori 3-1 and FTC-133 cell lysate proteins were separated under reduced conditions in 10% SDS-PAGE. Then the resolved proteins were electrotransferred onto a PVDF membrane (Millipore, Burlington, MA, USA) and incubated overnight in 2% BSA at 4 °C. The PVDF membranes were incubated for 1 h at RT with primary antibody anti-TPO (abcam, Cambridge, UK; ab203340) diluted 1:1000 in 2% BSA, and anti-GAPDH (Sigma-Aldrich, G9545) diluted 1:4000 in 2% BSA. After washing, the alkaline phosphatase (AP)-conjugated secondary antibody was used (diluted 1:4000): anti-mouse (Sigma-Aldrich, A2682) for TPO detection and anti-rabbit (Millipore, Burlington, MA, USA; AP304A) for GAPDH. The specific protein bands were visualized by colorimetric reaction after adding the substrates for AP: 5-bromo-4-chloro-3-indolyl phosphate (BCIP) and nitro blue tetrazolium (NBT) (Roche, Mannheim, Germany). The relative protein expression was quantified densitometrically using ImageLab software (Bio-Rad, Hercules, CA, USA). The experiment was performed in duplicate.
2.7. Flow Cytometry
The thyroid cells (5 × 104) were incubated with anti-TPO primary antibody (abcam, ab203340) diluted 1:200 in 50 µL PBS for 45 min at 4 °C. After washing in PBS and centrifugation (1200 rpm, 10 min, 4 °C), the cells were incubated with AlexaFluor488-conjugated anti-rabbit secondary antibody (Invitrogen, Paisley, UK; A21206) for 45 min at 4 °C in darkness. TPO-stained cells were analyzed quantitatively with a FACSCalibur flow cytometer (BD Biosciences, San Diego, CA, USA) using CellQuestPro software (BD Bioscience, San Diego, CA, USA). The experiment was performed in triplicate.
2.8. Lectin Blotting
Lectin staining was performed as previously described [
19].
Sambucus nigra agglutinin (SNA) specific for α2,6-linked sialic acid was used to determine the efficiency of IgG desialylation. Desialylated and untreated IgG (1 µg) from healthy donors and HT samples were separated on 10% SDS-PAGE stain free gels (Bio-Rad) in reducing conditions, followed by the electrotransfer onto a PVDF membrane (Millipore). The membranes were blocked with Carbo Free Blocking Solution (Vector Lab., Burlingame, CA, USA), overnight at 4 °C and incubated with biotinylated SNA (Vector Lab., B1305) diluted 1:4000 in TBS with ions (50 mM Tris-HCl, 150 mM NaCl, 1 mM MgCl
2, 1 mM CaCl
2, 1 mM MnCl
2, pH 7.5) for 1 h at RT. After washing in TBS, AP-conjugated ExtrAvidin (Sigma-Aldrich, E2636) diluted 1:4000 in TBS was applied. α2,6-sialylated IgG chains were visualized in AP colorimetric reaction as described above (
Section 2.6).
2.9. PBMC Isolation
Blood samples were obtained from healthy individuals. Blood was collected via venous puncture into EDTA collection tubes (S-Monovette, Sarstedt, Nümbrecht, Germany) on the day of the experiment. PBMCs were isolated by density gradient centrifugation using a Histopaque-1077 (Sigma-Aldrich, St. Louis, MO, USA; 10771). After washing twice in PBS, PBMCs were counted and resuspended in the appropriate culture medium.
2.10. ADCC Assay
Nthy-ori 3-1 and FTC-133 serving as target cells were harvested and seeded at a density of 1×104 cells per well into a 96-well black clear bottom plate a day before the experiment. On the day of the assay, the medium was removed, the cells were washed twice with PBS, and 50 µL of the appropriate fresh medium supplemented with 10% ultra-low IgG FBS (Gibco, Paisley, UK; A3381901) was added to each well. Desialylated and untreated IgG (30 µg) in 15 µL of PBS was added to the target cells and incubated for 2 h in a CO2 incubator at 37 °C. Then, PBMC and HL-60 effector cells resuspended in 35 µL of assay medium were added in the following proportions of target to effector cells: 1:50 for PBMC and 1:12 for HL-60. After 4 h of incubation in a CO2 incubator at 37 °C, target cell lysis was measured by detection of DNA released from the dead cells into the medium (CellTox Green Cytotoxicity Assay, Promega, Madison, WI, USA; G8742) according to the manufacturer’s instructions. Fluorescence was measured at 485 nm Ex/535 nm Em using an Infinite F200Pro plate reader (Tecan, Männedorf, Zürich, Switzerland). The experiment was performed in triplicate. A set of controls of spontaneous cell lysis was used, including target and effector cells without IgG, target cells and IgG without effector cells, only target cells, and only effector cells. Maximum cell lysis of both thyroid cell lines was measured after application of lysis solution supplied with the CellTox Green Cytotoxicity Assay.
2.11. CDC Assay
Nthy-ori 3-1 or FTC-133 target cells (1 × 10
4/well) were seeded into a 96-well black clear bottom plate on the day before the experiment. On the day of the assay, 50 µL of the appropriate fresh medium without FBS was added to the target cells after removing the medium and washing twice with PBS. Desialylated and untreated IgG (30 µg) in 15 µL of PBS was added to the target cells and incubated for 2 h in a CO
2 incubator at 37 °C. Normal human serum was used as complement source in the CDC reaction. Human serum at a final concentration of 10% or 25% (
v/
v) in culture medium was added to each well. After 4 h of incubation, thyroid cell lysis was determined using the CellTox Green Cytotoxicity Assay (Promega, Madison, WI, USA) as described above (
Section 2.10). CDC was repeated in four independent experiments.
2.12. Statistical Analysis
The expression of TPO in Nthy-ori 3-1 and FTC-133 cells was compared statistically using Student’s t-test. The differences in thyroid cell lysis triggered by control and HT IgGs in the ADCC model was analyzed statistically by two-way ANOVA followed by Tukey’s post hoc test using donor status (healthy or HT) and effector cell type (PBMC, HL-60) as the categorical variables. To compare the results obtained in the CDC model, two-way-ANOVA followed by Tukey’s post hoc test was applied. All of the statistical analyses were performed with Statistica 13.0 software (TIBCO Software, Palo Alto, CA, USA). p values < 0.05 were considered significant. The results are given as means of three or four independent experiments ± SD.
4. Discussion
The main purpose of the present research was to evaluate the possible effect of IgG
N-glycosylation on its effector functions in thyroid cell lysis, using established in vitro ADCC and CDC models of autoimmune thyroid destruction occurring in HT. We recently described the changes in IgG
N-glycosylation characteristic for AITD [
18] and the altered glycosylation profile of the rest of serum proteins in IgG-depleted sera from HT patients [
19]. These structural studies are the only ones that together show the whole serum glycome in HT. Besides our studies, anti-Tg glycosylation in HT donors was analyzed by Yuan and coworkers, who showed higher content of sialic acids, mannose, core fucose and Gal(β1,4)GlcNAc(β1,2)Man structures on anti-Tg from HT as compared to anti-Tg from healthy donors. The enhanced sialylation and fucosylation of anti-Tg were correlated with the serum level of this antibody in HT [
22]. These alterations of IgG glycosylation in HT prompted us to undertake the functional study and to develop the in vitro model to verify the hypothesis that altered IgG sialylation influences its effector function in thyroid autoimmunity. The present study used the whole pool of IgG instead of anti-TPO, known as the main inductor of ADCC and CDC in HT. The concentration of anti-TPO in serum is estimated to be up to 1.4 mg/mL [
23], and the titer of anti-TPO in healthy donor sera was drastically lower than in HT (
Table 1). As the result, it was impossible to isolate enough anti-TPO from healthy volunteer sera for the functional assays. Moreover, our previous results on altered glycosylation in AITD were obtained for the whole pool of IgG [
18]. The results described in the Introduction section show that serum or IgG isolated from AITD patients is more potent in inducing cell lysis in ADCC and CDC in vitro models. Our present work confirmed these results and for the first time demonstrated the impact of IgG glycosylation, altered in HT, in thyrocyte lysis.
Due to the use of a relatively non-invasive method of serum collection, analyses of IgG glycosylation became a popular tool in the search for glyco-biomarkers of chronic inflammatory diseases [
24,
25]. Studies of in vitro models showed that genetic or enzymatic rearrangements of IgG
N-glycans change its activity [
26,
27]. The vast majority of functional studies examined recombinant IgG molecules. The use of IgGs from HT and healthy donors with glycosylation altered during the autoimmune process gives our model a great advantage: it better reflects the pathological condition in the thyroid of HT patients, and the obtained results should serve as solid ground for further research.
Besides anti-TPO, the most common antibody present in over 90% of HT patients [
28], other cell membrane proteins can induce an autoimmune response (with lower incidence), such as sodium/iodide symporter (NIS) and pendrin [
29]. Anti-NIS and anti-pendrin IgGs were detected in 17% to 31% and 9% to 11% of AITD patients, respectively [
30]. Anti-thyroid IgGs other than anti-TPO, present in the whole pool of IgG used in our model, can contribute to the cytotoxicity effect. On the other hand, other non-autoantigenic IgGs present in the whole pool of isolated IgG might also affect the results of our experiments. Metcalfe and coworkers showed significantly lower cell lysis in the presence of HT than in the presence of GD sera, but there was no correlation between anti-TPO serum level and thyrocyte lysis, which could result from the activity of other anti-thyroid IgGs in ADCC and autoantibodies against thyroid antigens not characteristic only of AITD [
5]. To avoid the variability resulting from the presence of anti-thyroid IgGs other than anti-TPO in HT sera, we pooled the serum samples to each ADCC and CDC experiment and used the same amount of IgG per well.
The functional assays performed on ADCC and CDC models confirmed that IgGs isolated from HT patients induce thyroid cell lysis more efficiently (
Figure 4). This significant increase of thyrocyte lysis resulted primarily from the higher titer of anti-TPO in HT serum samples (
Table 1), despite having equal amounts of the whole pool of IgG in the HT and control variants used in both types of experiments. The statistically significant increase of thyroid cell lysis triggered by IgG from HT was observed only for HL-60 effector cells, not for PBMC. According to Rebuffat and coworkers, PBMC and unstimulated HL-60 cells express FcγRs (CD16, CD32, CD64) [
7], responsible for their effector function. The diverse cytotoxic effects caused by PBMC and HL-60 may result from the difference in the heterogeneity of these two cell populations. HL-60 is a homogenous cell population, in contrast to the diverse phenotype of PBMC. Although PBMC is a natural source of effector cells, the heterogeneity of this population may mask the effect of cell activation.
The functional study with Neu-treated IgG, designed to assess the contribution of IgG glycosylation to thyrocyte lysis, showed that desialylated IgGs from HT donors are more potent than control IgG without SA in inducing the ADCC reaction, while in the CDC model, we observed lower lysis of thyroid cells triggered by desialylated IgG from HT than in control samples. The opposite effects on thyrocyte lysis in the ADCC and CDC models with the use of desialylated IgG might result from a difference in Fc IgG binding to the C1q complement component or Fc receptor (FcR), the initial stage of CDC and ADCC, respectively (
Figure 5). Differences in effects in ADCC and CDC were also found by Quast and coworkers for sialylated therapeutic antibodies. Chemoenzymatic remodeling of
N-glycosylation on Rituximab (RTX), a commercial monoclonal antibody (mAb) that recognizes CD20 antigen on B cells, led to obtaining fully sialylated IgG. Neither the sialylated RTX nor the unsialylated glycoform of this mAb influenced B cell lysis and FcR binding efficiency in the ADCC model. On the other hand, sialylated RTX reduced the intensity of CDC. This impairment of effector function by sialylated RTX was associated with a decrease of Fc binding to C1q, which initiates the classical complement pathway [
31]. RTX was also used to determine an impact of Fc galactosylation on CDC. Galactosylation of IgG1 and IgG3 subclasses enhanced specific target cell lysis as a result of increased IgG binding to C1q, while the efficiency of galactosylated RTX in binding FcγRIIIa, necessary to activate the ADCC reaction, was unaffected [
32]. Opposite effects of mAb glycosylation on ADCC and CDC were also shown in the case of Fc
N-glycans with bisecting
N-acetylglucosamine (GlcNAc). An increase of ADCC activity and a minimal effect on CDC were observed after modification of RTX and Herceptin
N-glycans by adding bisecting GlcNAc. CDC was reduced by half with degalactosylated RTX in comparison to untreated IgG [
33]. Functional effects of
N-glycosylation remodeling on therapeutic mAb are well described, but the role of naturally occurring IgG with altered glycosylation in autoimmunity processes remains poorly understood.
To examine the impact of IgG glycosylation on its effector function, we compared the effect of thyrocyte lysis triggered by Neu-treated IgG to cell death induced by untreated IgG. The same enzymatic modification was applied in the ADCC assay to Pertuzumab, a therapeutic mAb, which recognizes human epidermal growth factor receptor 2 (HER2). A sixfold higher cytotoxic effect was observed in the case of sialylated and defucosylated Pertuzumab, and a 20-fold higher effect of cell lysis after desialylation and defucosylation of this mAb [
34]. These results showed that the effect of IgG desialylation strictly depends on the presence of core fucose. Enzymatic removal of core fucose from
N-glycans on native proteins is ineffective, because of the difficult access of fucosidase to a glycosidic bond linking fucose with an asparagine-linked GlcNAc in folded protein. In effect, the thyrocyte lysis in our models may have been influenced not only by a lack of IgG sialic acids but also by the presence of other components of IgG
N-glycans. It has been estimated that over 90% of serum IgG glycoforms are fucosylated; in the fucosylated IgG pool, three galactosylated groups can be distinguished in terms of the number of galactose residues: agalactosylated (~35%), monogalactosylated (~35%), and digalactosylated (~16%). The minority of IgGs are sialylated, only 5% to 10% of antibodies have a single SA residue, and under 1% are disialylated [
8,
35]. Although the percentage of sialylated IgG is relatively low, the role of IgG sialylation is crucial in the effector function [
8,
36]. The lack of terminal SA enhances IgG binding to FcR and ADCC intensity [
37]. The anti-inflammatory properties of sialylated IgG arise from an increased ability to bind DC-SIGN C-type lectin, which initiates inhibition of immune responses [
38]. In our study, enzymatic removal of SA from serum IgGs made the IgG pool uniform in terms of sialylation and exposed the terminal galactose residues. A decrease of IgG galactosylation has been observed in different autoimmune and inflammatory diseases; among them, the significance of this modification is best known in rheumatoid arthritis [
39,
40]. Attenuation of IgG galactosylation is associated with the reduction of antibody immunosuppressive potential [
41]. Besides exposure of Gal on IgG
N-glycans, the observed enhancement of the cytotoxic effect in our study may have resulted from the decrease of IgG core fucosylation in AITD patients, described previously by Martin and coworkers [
18].