2,3,4′,5-Tetrahydroxystilbene-2-O-β-D-Glucoside (THSG) Activates the Nrf2 Antioxidant Pathway and Attenuates Oxidative Stress-Induced Cell Death in Mouse Cochlear UB/OC-2 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Antibodies
2.2. Determination of Antioxidant Activity with the 1,1-Diphenyl-2-Picrylhydrazyl Radical 2,2-Diphenyl-1-(2,4,6-Trinitrophenyl)hydrazyl (DPPH) Radical Scavenging Assay
2.3. Cell Culture and Cell Viability Assay
2.4. Cell Fractionation
2.5. Western Blotting Analysis
2.6. Monodansylcadaverine (MDC) Staining
2.7. Transmission Electron Microscopy (TEM)
2.8. Annexin V/PI Staining
2.9. 5,5,6,6’-Tetrachloro-1,1’,3,3’-Tetraethylbenzimidazolylcarbocyanine Iodide (JC-1) Staining
2.10. Real-Time Polymerase Chain Reaction
2.11. Statistics
3. Results
3.2. H2O2 But Not 2,3,4′,5-Tetrahydroxystilbene-2-O-β-D-Glucoside Reduces Cell Viability
3.3. H2O2 Might Induce Apoptosis and Autophagy
3.4. 2,3,4′,5-Tetrahydroxystilbene-2-O-β-D-Glucoside Inhibits H2O2-Induced Autophagy
3.5. 2,3,4′,5-Tetrahydroxystilbene-2-O-β-D-Glucoside Protects against H2O2-Induced Apoptosis
3.6. 2,3,4′,5-Tetrahydroxystilbene-2-O-β-D-Glucoside Enhances Nrf2 Translocation into the Nucleus and Induces mRNA and Protein Expression of Antioxidant/Detoxifying Enzymes under H2O2-Induced Oxidative Stress Conditions
4. Discussion
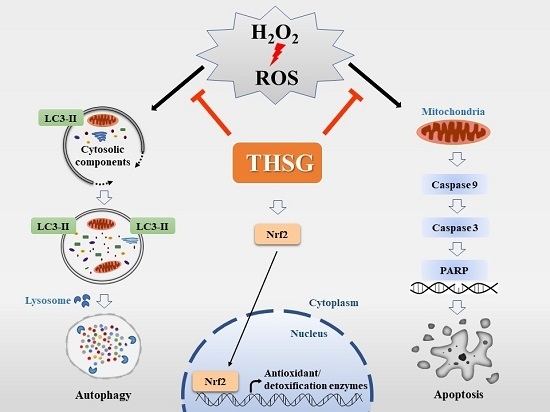
5. Conclusions
Author Contributions
Funding
Acknowledgments
References
- WHO. WHO Global Estimates on Prevalence of Hearing Loss. 2012. Available online: https://www.who.int/pbd/deafness/WHO_GE_HL.pdf (accessed on 17 November 2019).
- Mittal, R.; Nguyen, D.; Patel, A.P.; Debs, L.H.; Mittal, J.; Yan, D.; Eshraghi, A.A.; Van De Water, T.R.; Liu, X. Recent Advancements in the Regeneration of Auditory Hair Cells and Hearing Restoration. Front. Mol. Neurosci. 2017, 10, 236. [Google Scholar] [CrossRef] [Green Version]
- Kurabi, A.; Keithley, E.M.; Housley, G.D.; Ryan, A.F.; Wong, A.C.-Y. Cellular mechanisms of noise-induced hearing loss. Hear. Res. 2016, 349, 129–137. [Google Scholar] [CrossRef]
- Wu, H.-P.; Hsu, C.-J.; Cheng, T.-J.; Guo, Y.-L.L. N-acetylcysteine attenuates noise-induced permanent hearing loss in diabetic rats. Hear. Res. 2010, 267, 71–77. [Google Scholar] [CrossRef]
- Kros, C.J.; Steyger, P.S. Aminoglycoside- and Cisplatin-Induced Ototoxicity: Mechanisms and Otoprotective Strategies. Cold Spring Harb. Perspect. Med. 2018, 9, a033548. [Google Scholar] [CrossRef]
- Honkura, Y.; Matsuo, H.; Murakami, S.; Sakiyama, M.; Mizutari, K.; Shiotani, A.; Yamamoto, M.; Morita, I.; Shinomiya, N.; Kawase, T.; et al. NRF2 Is a Key Target for Prevention of Noise-Induced Hearing Loss by Reducing Oxidative Damage of Cochlea. Sci. Rep. 2016, 6, 19329. [Google Scholar] [CrossRef] [Green Version]
- Mandlekar, S.; Hong, J.-L.; Kong, A.-N.T. Modulation of metabolic enzymes by dietary phytochemicals: a review of mechanisms underlying beneficial versus unfavorable effects. Curr. Drug Metab. 2006, 7, 661–675. [Google Scholar] [CrossRef]
- Shen, G.; Xu, C.; Hu, R.; Jain, M.R.; Gopalkrishnan, A.; Nair, S.; Huang, M.-T.; Chan, J.Y.; Kong, A.-N.T. Modulation of nuclear factor E2-related factor 2-mediated gene expression in mice liver and small intestine by cancer chemopreventive agent curcumin. Mol. Cancer Ther. 2006, 5, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Shen, G.; Kong, A.-N. Nrf2 plays an important role in coordinated regulation of Phase II drug metabolism enzymes and Phase III drug transporters. Biopharm. Drug Dispos. 2009, 30, 345–55. [Google Scholar] [CrossRef] [Green Version]
- Ling, S.; Duan, J.; Ni, R.; Xu, J.-W. 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-glucoside Promotes Expression of the Longevity Gene Klotho. Oxidative Med. Cell. Longev. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ling, S.; Xu, J.-W. Biological Activities of 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-Glucoside in Antiaging and Antiaging-Related Disease Treatments. Oxidative Med. Cell. Longev. 2016, 2016, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Jin, M.L.; Wang, Z.; Park, G.; Choi, Y.-W. 2,3,4′,5-tetrahydroxystilbene-2-O-β-d-glucoside exerts anti-inflammatory effects on lipopolysaccharide-stimulated microglia by inhibiting NF-κB and activating AMPK/Nrf2 pathways. Food Chem. Toxicol. 2016, 97, 159–167. [Google Scholar] [CrossRef]
- Qin, S.; Hou, D.-X. Multiple regulations of Keap1/Nrf2 system by dietary phytochemicals. Mol. Nutr. Food Res. 2016, 60, 1731–1755. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ahn, S.-M.; Wang, Z.; Choi, Y.W.; Shin, H.K.; Choi, B.T. Neuroprotective effects of 2,3,5,4′-tetrahydoxystilbene-2-O-β-D-glucoside from Polygonum multiflorum against glutamate-induced oxidative toxicity in HT22 cells. J. Ethnopharmacol. 2017, 195, 64–70. [Google Scholar] [CrossRef]
- Motohashi, H.; Yamamoto, M. Nrf2–Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar] [CrossRef]
- Kim, Y.-R.; Baek, J.-I.; Kim, S.H.; Kim, M.-A.; Lee, B.; Ryu, N.; Kim, K.-H.; Choi, D.-G.; Kim, H.-M.; Murphy, M.P.; et al. Therapeutic potential of the mitochondria-targeted antioxidant MitoQ in mitochondrial-ROS induced sensorineural hearing loss caused by Idh2 deficiency. Redox Boil. 2018, 20, 544–555. [Google Scholar] [CrossRef]
- Huth, M.E.; Ricci, A.J.; Cheng, A.G. Mechanisms of Aminoglycoside Ototoxicity and Targets of Hair Cell Protection. Int. J. Otolaryngol. 2011, 2011, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Kiffin, R.; Bandyopadhyay, U.; Cuervo, A.M. Oxidative Stress and Autophagy. Antioxidants Redox Signal. 2006, 8, 152–162. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Zhai, N.; Chen, X.; Gan, F.; Li, H.; Huang, K. Ochratoxin A-Induced Apoptosis of IPEC-J2 Cells through ROS-Mediated Mitochondrial Permeability Transition Pore Opening Pathway. J. Agric. Food Chem. 2017, 65, 10630–10637. [Google Scholar] [CrossRef]
- Han, K.W.W.; Po, W.W.; Sohn, U.D.; Kim, H.-J. Benzyl Isothiocyanate Induces Apoptosis via Reactive Oxygen Species-Initiated Mitochondrial Dysfunction and DR4 and DR5 Death Receptor Activation in Gastric Adenocarcinoma Cells. Biomol. 2019, 9, 839. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.; Verma, S.S.; Rai, V.; Awasthee, N.; Arya, J.S.; Maiti, K.K.; Gupta, S.C. Curcuma raktakanda Induces Apoptosis and Suppresses Migration in Cancer Cells: Role of Reactive Oxygen Species. Biomolecules 2019, 9, 159. [Google Scholar] [CrossRef] [Green Version]
- Glick, D.; Barth, S.; MacLeod, K. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Lin, J.; Su, J.; Chen, X.; Jiang, P.; Huang, K. Glutamine Deficiency Promotes PCV2 Infection through Induction of Autophagy via Activation of ROS-Mediated JAK2/STAT3 Signaling Pathway. J. Agric. Food Chem. 2018, 66, 11757–11766. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-F.; Chin, H.-K.; Huang, W.-J.; Bai, L.-Y.; Huang, H.-Y.; Weng, J.-R. Induction of Apoptosis and Autophagy in Breast Cancer Cells by a Novel HDAC8 Inhibitor. Biomol. 2019, 9, 824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavakol, S.; Ashrafizadeh, M.; Deng, S.; Azarian, M.; Motavaf, M.; Poormoghadam, D.; Khanbabaei, H.; Afshar, E.G.; Mandegary, A.; Pardakhty, A.; et al. Autophagy Modulators: Mechanistic Aspects and Drug Delivery Systems. Biomolecules 2019, 9, 530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.; Wang, H.; Zhang, Z.; Liang, K. Stilbene glycoside protects osteoblasts against oxidative damage via Nrf2/HO-1 and NF-κB signaling pathways. Arch. Med Sci. 2018, 15, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.-Y.; Qin, X.-Y.; Yuan, M.-M.; Lu, G.-J.; Cheng, Y. 2,3,5,4′-Tetrahydroxystilbene-2-O-beta-D-glucoside Reverses Stress-Induced Depression via Inflammatory and Oxidative Stress Pathways. Oxidative Med. Cell. Longev. 2018, 2018, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-L.; Jeng, J.-H.; Wu, C.-C.; Hsieh, S.-L.; Huang, G.-C.; Leung, W.; Lee, C.-T.; Chen, C.-Y.; Lee, C.-H. Chemopreventive Potential of 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-glucoside on the Formation of Aberrant Crypt Foci in Azoxymethane-Induced Colorectal Cancer in Rats. BioMed Res. Int. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Zhang, S.-H.; Wang, W.-Q.; Wang, J.-L. Protective effect of tetrahydroxystilbene glucoside on cardiotoxicity induced by doxorubicin in vitro and in vivo. Acta Pharmacol. Sin. 2009, 30, 1479–1487. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Zhang, X.; Wu, H.; Zhou, Q.; Wang, Z.; Liu, R.; Liu, J.; Wang, X.; Hai, C. HO-1 Is Essential for Tetrahydroxystilbene Glucoside Mediated Mitochondrial Biogenesis and Anti-Inflammation Process in LPS-Treated RAW264.7 Macrophages. Oxidative Med. Cell. Longev. 2017, 2017, 1–13. [Google Scholar] [CrossRef]
- Ning, Z.; Li, Y.; Liu, N.; Orgah, J.O.; Zhu, J.; Wang, Y.; Zhu, Y. Tetrahydroxystilbene Glucoside Delayed Senile Symptoms in Old Mice via Regulation of the AMPK/SIRT1/PGC-1α Signaling Cascade. Gerontology 2018, 64, 457–465. [Google Scholar] [CrossRef]
- Vázquez, C.L.; Colombo, M.I. Chapter 6 Assays to Assess Autophagy Induction and Fusion of Autophagic Vacuoles with a Degradative Compartment, Using Monodansylcadaverine (MDC) and DQ-BSA. Method. Enzymol. 2009, 452, 85–95. [Google Scholar]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the Mitochondrial Membrane Potential Using the Cationic JC-1 Dye as a Sensitive Fluorescent Probe. BIO-PROTOCOL 2019, 9. [Google Scholar] [CrossRef] [PubMed]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.-Y.; Lin, J.-N.; Luo, Z.-Y.; Hsu, C.-J.; Wang, J.-S.; Wu, H.-P. 2,3,4′,5-Tetrahydroxystilbene-2-O-β-D-Glucoside (THSG) Activates the Nrf2 Antioxidant Pathway and Attenuates Oxidative Stress-Induced Cell Death in Mouse Cochlear UB/OC-2 Cells. Biomolecules 2020, 10, 465. https://doi.org/10.3390/biom10030465
Wu T-Y, Lin J-N, Luo Z-Y, Hsu C-J, Wang J-S, Wu H-P. 2,3,4′,5-Tetrahydroxystilbene-2-O-β-D-Glucoside (THSG) Activates the Nrf2 Antioxidant Pathway and Attenuates Oxidative Stress-Induced Cell Death in Mouse Cochlear UB/OC-2 Cells. Biomolecules. 2020; 10(3):465. https://doi.org/10.3390/biom10030465
Chicago/Turabian StyleWu, Tien-Yuan, Jia-Ni Lin, Zi-Yao Luo, Chuan-Jen Hsu, Jen-Shu Wang, and Hung-Pin Wu. 2020. "2,3,4′,5-Tetrahydroxystilbene-2-O-β-D-Glucoside (THSG) Activates the Nrf2 Antioxidant Pathway and Attenuates Oxidative Stress-Induced Cell Death in Mouse Cochlear UB/OC-2 Cells" Biomolecules 10, no. 3: 465. https://doi.org/10.3390/biom10030465
APA StyleWu, T. -Y., Lin, J. -N., Luo, Z. -Y., Hsu, C. -J., Wang, J. -S., & Wu, H. -P. (2020). 2,3,4′,5-Tetrahydroxystilbene-2-O-β-D-Glucoside (THSG) Activates the Nrf2 Antioxidant Pathway and Attenuates Oxidative Stress-Induced Cell Death in Mouse Cochlear UB/OC-2 Cells. Biomolecules, 10(3), 465. https://doi.org/10.3390/biom10030465