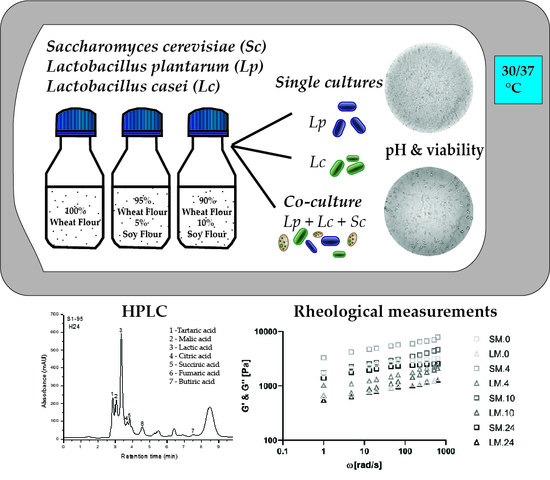
Exploitation of Lactic Acid Bacteria and Baker’s Yeast as Single or Multiple Starter Cultures of Wheat Flour Dough Enriched with Soy Flour
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microorganisms and Culture Conditions
2.3. Sourdough Preparation
2.4. Fermentations
2.5. Organic Acid and Secondary Metabolite Analysis by HPLC
2.6. pH Measurements
2.7. Rheological Measurements
2.8. Statistical Analysis
3. Results and Discussions
3.1. pH and Cell Viability
3.2. Rheological Measurements
3.3. Organic Acid and Secondary Metabolite Analysis by HPLC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, Z.; Mu, T.; Sun, H. Microbial characterization of five Chinese traditional sourdoughs by high-throughput sequencing and their impact on the quality of potato steamed bread. Food Chem. 2019, 274, 710–717. [Google Scholar] [CrossRef]
- Winters, M.; Panayotides, D.; Bayrak, M.; Rémont, G.; Viejo, C.G.; Liu, D.; Le, B.; Liu, Y.; Luo, J.; Zhang, P.; et al. Defined co-cultures of yeast and bacteria modify the aroma, crumb and sensory properties of bread. J. Appl. Microbiol. 2019, 127, 778–793. [Google Scholar] [CrossRef] [PubMed]
- Corona, O.; Alfonzo, A.; Ventimiglia, G.; Nasca, A.; Francesca, N.; Martorana, A.; Moschetti, G.; Settanni, L. Industrial application of selected lactic acid bacteria isolated from local semolinas for typical sourdough bread production. Food Microbiol. 2016, 59, 43–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maidana, S.D.; Ficoseco, C.A.; Bassi, D.; Cocconcelli, P.S.; Puglisi, E.; Savoy, G.; Vignolo, G.; Fontana, C. Biodiversity and technological-functional potential of lactic acid bacteria isolated from spontaneously fermented chia sourdough. Int. J. Food Microbiol. 2020, 316, 108425. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Y.; Yang, Y.; Yi, H.; Zhang, L.; He, G. The influence of different lactic acid bacteria on sourdough flavor and a deep insight into sourdough fermentation through RNA sequencing. Food Chem. 2020, 307, 125529. [Google Scholar] [CrossRef]
- Montemurro, M.; Coda, R.; Rizzello, C.G. Recent Advances in the Use of Sourdough. Foods 2019, 8, 129. [Google Scholar] [CrossRef] [Green Version]
- Mironeasa, S.; Mironeasa, C. Dough bread from refined wheat flour partially replaced by grape peels: Optimizing the rheological properties. J. Food Process. Eng. 2019, 42, 1–14. [Google Scholar] [CrossRef]
- Călinoiu, L.F.; Vodnar, D.C. Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Badger, T.M.; Ronis, M.J.J.; Wu, X. Non-Isoflavone phytochemicals in soy and their health effects. J. Agric. Food Chem. 2010, 58, 8119–8133. [Google Scholar] [CrossRef]
- Liu, Y.; Hilakivi-Clarke, L.; Zhang, Y.; Wang, X.; Pan, Y.X.; Xuan, J.; Fleck, S.C.; Doerge, D.R.; Helferich, W.G. Isoflavones in soy flour diet have different effects on whole-genome expression patterns than purified isoflavone mix in human MCF-7 breast tumors in ovariectomized athymic nude mice. Mol. Nutr. Food Res. 2015, 59, 1419–1430. [Google Scholar] [CrossRef]
- Ribotta, P.D.; León, A.E.; Pérez, G.T.; Añón, M.C. Electrophoresis studies for determining wheat-soy protein interactions in dough and bread. Eur. Food Res. Technol. 2005, 221, 48–53. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, Z.; Huang, W.; Omedi, J.O.; Wang, F.; Zou, Q.; Zheng, J. Isoflavone aglycones enrichment in soybean sourdough bread fermented by Lactic acid bacteria strains isolated from traditional Qu starters: Effects on in-vitro gastrointestinal digestion, nutritional, and baking properties. Cereal Chem. 2018, 96, 129–141. [Google Scholar] [CrossRef]
- Ryan, K.J.; Homco-Ryan, C.L.; Jenson, J.; Robbins, K.L.; Prestat, C.; Brewer, M.S. Lipid extraction process on texturized soy flour and wheat gluten protein-protein interactions in a dough matrix. Cereal Chem. 2002, 79, 434–438. [Google Scholar] [CrossRef]
- Bojňanská, T.; Šmitalová, J.; Vollmannová, A.; Tokár, M.; Vietoris, V. Bakery products with the addition of soybean flour and their quality after freezer storage of dough. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 18–22. [Google Scholar] [CrossRef]
- Friedman, M.; Brandon, D.L. Nutritional and health benefits of soy proteins. J. Agric. Food Chem. 2001, 49, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Faraj, A.; Vasanthan, T. Soybean Isoflavones: Effects of Processing and Health Benefits. Food Rev. Int. 2004, 20, 51–75. [Google Scholar] [CrossRef]
- Besler, M.; Helm, R.M.; Ogawa, T. Soybean (Glycine max L.) Allergen Data Collection—Update: Internet Symp. Food Allerg. 2000, 2, 1–35. [Google Scholar]
- Aguirre, L.; Hebert, E.M.; Garro, M.S.; de Giori, G.S. Proteolytic activity of Lactobacillus strains on soybean proteins. LWT-Food Sci. Technol. 2014, 59, 780–785. [Google Scholar] [CrossRef]
- Simmons, A.L.; Smith, K.B.; Vodovotz, Y. Soy ingredients stabilize bread dough during frozen storage. J. Cereal Sci. 2015, 56, 232–238. [Google Scholar] [CrossRef]
- De Vuyst, L.; Neysens, P. The sourdough microflora: Biodiversity and metabolic interactions. Trends Food Sci. Technol. 2005, 16, 43–56. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Lorusso, A.; Montemurro, M.; Gobbetti, M. Use of sourdough made with quinoa (Chenopodium quinoa) flour and autochthonous selected lactic acid bacteria for enhancing the nutritional, textural and sensory features of white bread. Food Microbiol. 2016, 56, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Poutanen, K.; Flander, L.; Katina, K. Sourdough and cereal fermentation in a nutritional perspective. Food Microbiol. 2009, 26, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.I.; Arendt, E.K. A Review of the Application of Sourdough Technology to Wheat Breads. Adv. Food Nutr. Res. 2005, 49, 137–161. [Google Scholar] [PubMed]
- Östman, E. Fermentation as a Means of Optimizing the Glycaemic Index Food Mechanisms and Metabolic Merits with Emphasis on Lactic Acid in Cereal Products; Lund Institute of Technology: Lund, Sweden, 2003; ISBN 9174220160. [Google Scholar]
- Foster-Powell, K.; Holt, S.H.A.; Brand-Miller, J.C. International Table of Glycemic Index and Glycemic Load values. Am. J. Clin. Nutr. 2002, 31, 5–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Martău, G.A.; Coman, V.; Vodnar, D.C. Recent advances in the biotechnological production of erythritol and mannitol. Crit. Rev. Biotechnol. 2020, 1–15. [Google Scholar] [CrossRef]
- Calinoiu, L.; Vodnar, D.; Precup, G. The Probiotic Bacteria Viability under Different Conditions. Bull. UASVM Food Sci. Technol. 2014, 73, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.W.; Liong, M.T.; Tsai, Y.C. New perspectives of Lactobacillus plantarum as a probiotic: The gut-heart-brain axis. J. Microbiol. 2018, 56, 601–613. [Google Scholar] [CrossRef]
- Hui, Y.H.; Corke, H.; De Leyn, I.; Nip, W.-K.; Cross, N. Bakery Products Science and Technology, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2006; ISBN 9780813801872. [Google Scholar]
- Abedfar, A.; Hosseininezhad, M.; Corsetti, A. Effect of wheat bran sourdough with exopolysaccharide producing Lactobacillus plantarum (NR_104573.1)on quality of pan bread during shelf life. LWT 2019, 111, 158–166. [Google Scholar] [CrossRef]
- Gobbetti, M.; Gänzle, M. Handbook on Sourdough Biotechnology; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 9781461454250. [Google Scholar]
- Ly, D.; Mayrhofer, S.; Yogeswara, I.B.A.; Nguyen, T.H.; Domig, K.J. Identification, classification and screening for γ-amino-butyric acid production in lactic acid bacteria from cambodian fermented foods. Biomolecules 2019, 9, 768. [Google Scholar] [CrossRef] [Green Version]
- Hill, D.; Sugrue, I.; Tobin, C.; Hill, C.; Stanton, C.; Ross, R.P. The Lactobacillus casei group: History and health related applications. Front. Microbiol. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reale, A.; Renzo, T.D.; Zotta, T.; Preziuso, M.; Boscaino, F.; Ianniello, R.; Storti, L.V.; Tremonte, P.; Coppola, R. Effect of respirative cultures of Lactobacillus casei on model sourdough fermentation. LWT-Food Sci. Technol. 2016, 73, 622–629. [Google Scholar] [CrossRef]
- Ryan, L.A.M.; Zannini, E.; Bello, F.D.; Pawlowska, A.; Koehler, P.; Arendt, E.K. Lactobacillus amylovorus DSM 19280 as a novel food-grade antifungal agent for bakery products. Int. J. Food Microbiol. 2011, 146, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Tyler, C.A.; Kopit, L.; Doyle, C.; Yu, A.O.; Hugenholtz, J.; Marco, M.L. Polyol production during heterofermentative growth of the plant isolate Lactobacillus florum 2F. J. Appl. Microbiol. 2016, 120, 1336–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, D.M.; Bae, J.H.; Kim, M.S.; Kim, H.; Kang, S.D.; Shim, S.; Lee, D.; Seo, J.H.; Kang, H.; Han, N.S. Suitability of Lactobacillus plantarum SPC-SNU 72-2 as a Probiotic Starter for Sourdough Fermentation. J. Microbiol. Biotechnol. 2019, 29, 1729–1738. [Google Scholar] [CrossRef]
- Sieuwerts, S.; Bron, P.A.; Smid, E.J. Mutually stimulating interactions between lactic acid bacteria and Saccharomyces cerevisiae in sourdough fermentation. LWT-Food Sci. Technol. 2018, 90, 201–206. [Google Scholar] [CrossRef]
- Lavermicocca, P.; Valerio, F.; Evidente, A.; Lazzaroni, S.; Corsetti, A.; Gobbetti, M. Purification and characterization of novel antifungal compounds from the sourdough Lactobacillus plantarum strain 21B. Appl. Environ. Microbiol. 2000, 66, 4084–4090. [Google Scholar] [CrossRef] [Green Version]
- Mitrea, L.; Ranga, F.; Fetea, F.; Dulf, F.V.; Rusu, A.; Trif, M.; Vodnar, D.C. Biodiesel-Derived Glycerol Obtained from Renewable Biomass—A Suitable Substrate for the Growth of Candida zeylanoides Yeast Strain ATCC 20367. Microorganisms 2019, 7, 265. [Google Scholar] [CrossRef] [Green Version]
- Renschler, M.A.; Wyatt, A.; Anene, N.; Robinson-Hill, R.; Pickerill, E.S.; Fox, N.E.; Griffith, J.A.; McKillip, J.L. Using nitrous acid-modified de Man, Rogosa, and Sharpe medium to selectively isolate and culture lactic acid bacteria from dairy foods. J. Dairy Sci. 2020, 103, 1215–1222. [Google Scholar] [CrossRef]
- Vodnar, D.C.; Socaciu, C.; Rotar, A.M.; Stãnilã, A. Morphology, FTIR fingerprint and survivability of encapsulated lactic bacteria (Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus) in simulated gastric juice and intestinal juice. Int. J. Food Sci. Technol. 2010, 45, 2345–2351. [Google Scholar] [CrossRef]
- Szabo, K.; Dulf, F.V.; Diaconeasa, Z.; Vodnar, D.C. Antimicrobial and antioxidant properties of tomato processing byproducts and their correlation with the biochemical composition. LWT-Food Sci. Technol. 2019, 116, 108858. [Google Scholar] [CrossRef]
- Călinoiu, L.-F.; Catoi, A.-F.; Vodnar, D.C. Solid-State Yeast Fermented Wheat and Oat Bran as A Route for Delivery of Antioxidants. Antioxidants 2019, 8, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitrea, L.; Trif, M.; Vodnar, D.-C. The effect of crude glycerol impurities on 1,3-propanediol biosynthesis by Klebsiella pneumoniae DSMZ 2026. Renew. Energy 2020, 153, 1418–1427. [Google Scholar]
- Szabo, K.; Teleky, B.E.; Mitrea, L.; Călinoiu, L.F.; Martău, G.A.; Simon, E.; Varvara, R.A.; Vodnar, D.C. Active packaging-poly (vinyl alcohol) films enriched with tomato by-products extract. Coatings 2020, 10, 141. [Google Scholar] [CrossRef] [Green Version]
- Mitrea, L.; Călinoiu, L.-F.; Martău, G.-A.; Szabo, K.; Teleky, B.-E.; Mureșan, V.; Rusu, A.-V.; Socol, C.-T.; Vodnar, D.-C. Poly(vinyl alcohol)-Based Biofilms Plasticized with Polyols and Colored with Pigments Extracted from Tomato By-Products. Polymers 2020, 12, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paucean, A.; Vodnar, D.C.; Socaci, S.A.; Socaciu, C. Carbohydrate metabolic conversions to lactic acid and volatile derivatives, as influenced by Lactobacillus plantarum ATCC 8014 and Lactobacillus casei ATCC 393 efficiency during in vitro and sourdough fermentation. Eur. Food Res. Technol. 2013, 237, 679–689. [Google Scholar] [CrossRef]
- Aguirre, L.; Garro, M.S.; de Giori, G.S. Enzymatic hydrolysis of soybean protein using lactic acid bacteria. Food Chem. 2008, 111, 976–982. [Google Scholar] [CrossRef]
- Robert, H.; Gabriel, V.; Lefebvre, D.; Rabier, P.; Vayssier, Y.; Fontagné-Faucher, C. Study of the behaviour of Lactobacillus plantarum and Leuconostoc starters during a complete wheat sourdough breadmaking process. LWT-Food Sci. Technol. 2006, 39, 256–265. [Google Scholar] [CrossRef]
- Sun, L.; Li, X.; Zhang, Y.; Yang, W.; Ma, G.; Ma, N.; Hu, Q.; Pei, F. A novel lactic acid bacterium for improving the quality and shelf life of whole wheat bread. Food Control. 2020, 109, 106914. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Albrecht, D.; Bomser, J.; Schwartz, S.J.; Vodovotz, Y. Isoflavone Profile and Biological Activity of Soy Bread. J. Agric. Food Chem. 2003, 51, 7611–7616. [Google Scholar] [CrossRef]
- Fessas, D.; Schiraldi, A. Water properties in wheat flour dough. I: Classical thermogravimetry approach. Food Chem. 2001, 72, 237–244. [Google Scholar] [CrossRef]
- Hardt, N.A.; Boom, R.M.; van der Goot, A.J. Wheat dough rheology at low water contents and the influence of xylanases. Food Res. Int. 2014, 66, 478–484. [Google Scholar] [CrossRef]
- Sun, X.; Koksel, F.; Nickerson, M.T.; Scanlon, M.G. Modeling the viscoelastic behavior of wheat flour dough prepared from a wide range of formulations. Food Hydrocoll. 2020, 98, 105129. [Google Scholar] [CrossRef]
- Arendt, E.K.; Ryan, L.A.M.; Bello, F.D. Impact of sourdough on the texture of bread. Food Microbiol. 2007, 24, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, J.; Tang, X. Effects of whey and soy protein addition on bread rheological property of wheat flour. J. Texture Stud. 2018, 49, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.M. Gobbetti. Trends Food Sci. Technol. 1998, 9. [Google Scholar]
- Rehm, H.J.; Reed, G. Book Review: Book Review. Acta Biotechnol. 1985, 5, 451–454. [Google Scholar]
- Vodnar, D.C.; Paucean, A.; Dulf, F.V.; Socaciu, C. HPLC characterization of lactic acid formation and FTIR fingerprint of probiotic bacteria during fermentation processes. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 109–113. [Google Scholar]
- Corsetti, A.; Gobbetti, M.; Rossi, J.; Damiani, P. Antimould activity of sourdough lactic acid bacteria: Identification of a mixture of organic acids produced by Lactobacillus sanfrancisco CB1. Appl. Microbiol. Biotechnol. 1998, 50, 253–256. [Google Scholar] [CrossRef]
- Gourama, H. Inhibition of growth and mycotoxin production of Penicillium by Lactobacillus species. LWT-Food Sci. Technol. 1997, 30, 279–283. [Google Scholar] [CrossRef]
- Niku-Paavola, M.L.; Laitila, A.; Mattila-Sandholm, T.; Haikara, A. New types of antimicrobial compounds produced by Lactobacillus plantarum. J. Appl. Microbiol. 1999, 86, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Plessas, S.; Fisher, A.; Koureta, K.; Psarianos, C.; Nigam, P.; Koutinas, A.A. Application of Kluyveromyces marxianus, Lactobacillus delbrueckii ssp. bulgaricus and L. helveticus for sourdough bread making. Food Chem. 2008, 106, 985–990. [Google Scholar]
- Kaneuchi, C.; Seki, M.; Komagata, K. Production of Succinic Acid from Citric Acid and Related Acids by Lactobacillus Strains. Appl. Environ. Microbiol. 1988, 54, 3053–3056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-Muñiz, I.; Banavara, D.S.; Budinich, M.F.; Rankin, S.A.; Dudley, E.G.; Steele, J.L. Lactobacillus casei metabolic potential to utilize citrate as an energy source in ripening cheese: A bioinformatics approach. J. Appl. Microbiol. 2006, 101, 872–882. [Google Scholar] [CrossRef]
- Mozzi, F.; Ortiz, M.E.; Bleckwedel, J.; De Vuyst, L.; Pescuma, M. Metabolomics as a tool for the comprehensive understanding of fermented and functional foods with lactic acid bacteria. Food Res. Int. 2013, 54, 1152–1161. [Google Scholar] [CrossRef]
- Zelle, R.M.; De Hulster, E.; Van Winden, W.A.; De Waard, P.; Dijkema, C.; Winkler, A.A.; Geertman, J.M.A.; Van Dijken, J.P.; Pronk, J.T.; Van Maris, A.J.A. Malic acid production by Saccharomyces cerevisiae: Engineering of pyruvate carboxylation, oxaloacetate reduction, and malate export. Appl. Environ. Microbiol. 2008, 74, 2766–2777. [Google Scholar] [CrossRef] [Green Version]
- Gänzle, M.G. Enzymatic and bacterial conversions during sourdough fermentation. Food Microbiol. 2014, 37, 2–10. [Google Scholar] [CrossRef]
- Hu, C.H.; Ren, L.Q.; Zhou, Y.; Ye, B.C. Characterization of antimicrobial activity of three Lactobacillus plantarum strains isolated from Chinese traditional dairy food. Food Sci. Nutr. 2019, 7, 1997–2005. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, A.; Okada, S.; Hols, P.; Satoh, E. Metabolic engineering of Lactobacillus plantarum for succinic acid production through activation of the reductive branch of the tricarboxylic acid cycle. Enzym. Microb. Technol. 2013, 53, 97–103. [Google Scholar] [CrossRef]




| Sourdough Fermentation Batch | M.M. | A | B | C | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH range | C.c. (CFU/g) | W.B. (g/L) | pH range | C.c. (CFU/g) | pH range | C.c. (CFU/g) | pH range | C.c. (CFU/g) | ||
| S1 | Initial | 6.39 ± 0.12 | 1 × 108 | 0.0036 ± 0.001 | 6.12 ± 0.19 | 3.0 × 106 | 6.07 ± 0.22 | 2.0 × 106 | 6.02 ± 0.09 | 2.2 × 106 |
| Final | 3.58 ± 0.23 | 4.3 × 1010 | 0.0172 ± 0.004 | 3.79 ± 0.16 | 4.8 × 109 | 3.81 ± 0.25 | 3.3 × 109 | 3.64 ± 0.27 | 4.1 × 109 | |
| S2 | Initial | 6.41 ± 0.34 | 3.5 × 108 | 0.0054 ± 0.002 | 6.15 ± 0.21 | 3.0 × 105 | 5.62 ± 0.17 | 8.0 × 105 | 5.73 ± 0.35 | 2.0 × 105 |
| Final | 3.56 ± 0.19 | 9.5 × 1012 | 0.0159 ± 0.007 | 3.81 ± 0.08 | 1.5 × 109 | 3.34 ± 0.10 | 3.5 × 109 | 3.35 ± 0.15 | 5.7 × 109 | |
| S3 | Initial LAB | 6.37 ± 0.12 | 2.0 × 108 | 0.0069 ± 0.001 | 6.19 ± 0.09 | 3.0 × 105 | 5.14 ± 0.05 | 1.5 × 105 | 5.09 ± 0.12 | 3.0 × 105 |
| Final LAB | 3.36 ± 0.32 | 1.5 × 1012 | 0.0156 ± 0.003 | 3.92 ± 0.11 | 1.1 × 109 | 3.55 ± 0.30 | 4.2 × 109 | 3.50 ± 0.26 | 2.9 × 109 | |
| Initial Sc | 6.37 ± 0.12 | 4.7 × 106 | 0.0069 ± 0.001 | 6.19 ± 0.09 | 1.7 × 103 | 5.14 ± 0.05 | 2.4 × 103 | 5.09 ± 0.12 | 1.9 × 103 | |
| Final Sc | 3.36 ± 0.32 | 1.8 × 108 | 0.0156 ± 0.003 | 3.92 ± 0.11 | 1.8 × 107 | 3.55 ± 0.30 | 2.6 × 107 | 3.50 ± 0.26 | 4.1 × 107 | |
| Org. Acids (g/L) | Lactic A. | Acetic A. | Malic A. | Succinic A. | Tartaric A. | Citric A. | Fumaric A. | |
|---|---|---|---|---|---|---|---|---|
| Substr. | Time (h) | |||||||
| MM | 0 | n.d. | 0.663 ± 0.07 a | 1.384 ± 0.05 a | 6.609 ± 0.14 a | 0.342 ± 0.06 a | 3.081 ± 0.07 a | 0.033 ± 0.07 a |
| 4 | 1.812 ± 0.04 a | 1.812 ± 0.09 a | 2.139 ± 0.06 a | 9.496 ± 0.10 a | 0.668 ± 0.07 a | 3.430 ± 0.08 a | 0.015 ± 0.02 a | |
| 10 | 2.899 ± 0.06 a | 2.408 ± 0.10 a | 1.641 ± 0.04 a | 8.259 ± 0.08 a | 0.478 ± 0.08 a | 1.630 ± 0.08 a | 0.017 ± 0.01 a | |
| 24 | 7.856 ± 0.06 a | 2.899 ± 0.09 a | 1.557 ± 0.09 a | 8.361 ± 0.11 a | 0.451 ± 0.12 a | 0.813 ± 0.05 a | 0.017 ± 0.02 a | |
| A | 0 | 0.363 ± 0.08 a | n.d. | 0.482 ± 0.06 b | 0.673 ± 0.08 c | 0.152 ± 0.05 b | n.d. | 0.027 ± 0.06 a |
| 4 | 0.462 ± 0.07 b | n.d. | 0.494 ± 0.07 c | 1.077 ± 0.08 d | 0.169 ± 0.07 b | n.d. | 0.029 ± 0.04 a | |
| 10 | 0.643 ± 0.08 c | n.d. | 0.570 ± 0.06 b | 0.572 ± 0.04 d | 0.186 ± 0.02 b | n.d. | 0.013 ± 0.04 a | |
| 24 | 1.514 ± 0.06 d | n.d. | 0.468 ± 0.10 b | 0.130 ± 0.03 d | 0.100 ± 0.08 b | 0.212 ± 0.03 b | 0.008 ± 0.02 a | |
| B | 0 | n.d. | n.d. | 0.612 ± 0.09 b | 1.286 ± 0.04 b | 0.354 ± 0.06 a | 0.414 ± 0.05 b | 0.037 ± 0.08 a |
| 4 | n.d. | n.d. | 0.619 ± 0.11 b,c | 1.437 ± 0.09 c | 0.339 ± 0.06 ab | 0.371 ± 0.05 c | 0.037 ± 0.07 a | |
| 10 | 1.328 ± 0.06 b,c | n.d. | 1.323 ± 0.04 a | 1.918 ± 0.03 c | 0.459 ± 0.04 a | 0.532 ± 0.08 b | 0.034 ± 0.06 a | |
| 24 | 3.787 ± 0.03 b | n.d. | 1.025 ± 0.09 a | 0.785 ± 0.08 c | 0.205 ± 0.07 b | 0.295 ± 0.07 b | 0.007 ± 0.01 a | |
| C | 0 | n.d. | n.d. | 0.457 ± 0.13 b | 0.531 ± 0.07 c | 0.181 ± 0.07 b | 0.450 ± 0.06 b | 0.027 ± 0.05 a |
| 4 | n.d. | n.d. | 1.471 ± 0.08 b | 3.993 ± 0.05 b | 0.589 ± 0.08 a | 0.979 ± 0.06 b | 0.024 ± 0.03 a | |
| 10 | 1.634 ± 0.12 b | n.d. | 1.393 ± 0.03 a | 3.564 ± 0.08 b | 0.598 ± 0.07 a | 0.648 ± 0.08 b | 0.010 ± 0.02 a | |
| 24 | 3.330 ± 0.01 c | n.d. | 1.004 ± 0.07 a | 1.070 ± 0.06 b | 0.393 ± 0.13 a | 0.362 ± 0.09 b | 0.001 ± 0.00 a | |
| Org. Acids (g/L) | Lactic A. | Acetic A. | Malic A. | Succinic A. | Tartaric A. | Citric A. | Fumaric A. | |
|---|---|---|---|---|---|---|---|---|
| Substr. | Time (h) | |||||||
| MM | 0 | 2.496 ± 0.04 a | 3.571 ± 0.11 a | 1.225 ± 0.04 a | 3.661 ± 0.11 a | 0.371 ± 0.07 a | 2.930 ± 0.04 a | 0.029 ± 0.05 a |
| 4 | 3.139 ± 0.08 a | 4.081 ± 0.09 a | 1.367 ± 0.14 a | 8.448 ± 0.09 a | 0.463 ± 0.08 a | 3.141 ± 0.07 a | 0.020 ± 0.09 a | |
| 10 | 4.972 ± 0.10 a | 3.062 ± 0.06 a | 1.831 ± 0.05 a | 9.244 ± 0.09 a | 0.558 ± 0.06 a | 3.174 ± 0.07 a | 0.014 ± 0.07 a | |
| 24 | 8.329 ± 0.04 a | 2.369 ± 0.12 a | 1.870 ± 0.08 a | 3.787 ± 0.08 a | 0.495 ± 0.05 a | 1.091 ± 0.07 a | 0.015 ± 0.04 a | |
| A | 0 | n.d. | n.d. | 0.402 ± 0.06 b | n.d. | 0.099 ± 0.03 b | 0.225 ± 0.06 c | 0.023 ± 0.01 a |
| 4 | n.d. | n.d. | 0.518 ± 0.11 b | n.d. | 0.325 ± 0.06 a | 1.052 ± 0.10 b | 0.024 ± 0.12 a | |
| 10 | 0.931 ± 0.12 b | n.d. | 0.473 ± 0.07 b | n.d. | 0.297 ± 0.06 a,b | 0.242 ± 0.09 b | 0.022 ± 0.08 a | |
| 24 | 2.137 ± 0.01 b | n.d. | 0.737 ± 0.07 b | n.d. | 0.259 ± 0.05 a,b | 0.521 ± 0.10 b | 0.033 ± 0.06 a | |
| B | 0 | n.d. | n.d. | 0.451 ± 0.07 b | n.d. | 0.217 ± 0.07 a,b | 0.559 ± 0.07 b | 0.025 ± 0.04 a |
| 4 | 0.534 ± 0.07 b | n.d. | 0.966 ± 0.05 a,b | n.d. | 0.301 ± 0.09 a | 0.808 ± 0.08 b,c | 0.032 ± 0.02 a | |
| 10 | 0.149 ± 0.05 b | n.d. | 0.491 ± 0.07 b | n.d. | 0.303 ± 0.04 a,b | 0.364 ± 0.10 b | 0.010 ± 0.01 a | |
| 24 | 1.878 ± 0.04 c | n.d. | 0.666 ± 0.12 b | n.d. | 0.205 ± 0.04 b | 0.273 ± 0.08 b | 0.003 ± 0.01 a | |
| C | 0 | n.d. | n.d. | 0.451 ± 0.06 b | n.d. | 0.126 ± 0.06 b | 0.176 ± 0.7 c | 0.020 ± 0.04 a |
| 4 | n.d. | n.d. | 0.506 ± 0.04 b | n.d. | 0.200 ± 0.05 a | 0.427 ± 0.08 c | 0.031 ± 0.07 a | |
| 10 | 0.349 ± 0.08 b | n.d. | 0.432 ± 0.11 b | n.d. | 0.128 ± 0.02 b | 0.231 ± 0.10 b | 0.001 ± 0.00 a | |
| 24 | 2.212 ± 0.11 b | n.d. | 0.657 ± 0.05 b | n.d. | 0.456 ± 0.08 a | 1.133 ± 0.11 a | 0.004 ± 0.01 a | |
| Org. Acids (g/L) | Lactic A. | Acetic A. | Malic A. | Succinic A. | Tartaric A. | Citric A. | Fumaric A. | |
|---|---|---|---|---|---|---|---|---|
| Substr. | Time (h) | |||||||
| MM | 0 | 2.079 ± 0.09 a | 2.870 ± 0.10 a | 1.501 ± 0.10 a | 8.057 ± 0.09 a | 0.407 ± 0.08 a | 4.604 ± 0.08 a | 0.016 ± 0.04 a |
| 4 | 3.267 ± 0.08 a | 4.004 ± 0.05 a | 1.689 ± 0.09 a | 9.681 ± 0.11 a | 0.517 ± 0.11 a | 3.688 ± 0.08 a | 0.013 ± 0.07 a | |
| 10 | 4.715 ± 0.06 a | 4.147 ± 0.08 a | 1.724 ± 0.09 a | 7.907 ± 0.07 a | 0.387 ± 0.09 a | 1.814 ± 0.12b | 0.021 ± 0.04 a | |
| 24 | 4.935 ± 0.07 a | 4.918 ± 0.07 a | 0.934 ± 0.1 a | 8.094 ± 0.18 | 0.395 ± 0.07 a | 1.996 ± 0.06 a | 0.021 ± 0.09 a | |
| A | 0 | n.d. | n.d. | 0.359 ± 0.07 c | n.d. | 0.436 ± 0.04 a | 0.391 ± 0.05 c | 0.044 ± 0.02 a |
| 4 | n.d. | n.d. | 0.682 ± 0.06 a,b | n.d. | 0.556 ± 0.10 a | 1.244 ± 0.07 b | 0.049 ± 0.07 a | |
| 10 | 0.578 ± 0.06 b | n.d. | 0.838 ± 0.03 b | n.d. | 0.203 ± 0.02 b | 0.389 ± 0.09 c | 0.037 ± 0.08 a | |
| 24 | 2.178 ± 0.03 c | 0.022 ± 0.03 d | 0.575 ± 0.11 b | n.d. | 0.176 ± 0.08 b | 0.198 ± 0.09 c | 0.010 ± 0.03 a | |
| B | 0 | n.d. | n.d. | 0.675 ± 0.10 b | n.d. | 0.314 ± 0.11 a | 0.752 ± 0.13 b | 0.051 ± 0.10 a |
| 4 | n.d. | n.d. | 0.355 ± 0.04 b | n.d. | 0.116 ± 0.04 b | 0.630 ± 0.08 b | 0.020 ± 0.08 a | |
| 10 | 0.899 ± 0.06 b | n.d. | 1.152 ± 0.06 b | n.d. | 0.252 ± 0.01 a,b | 0.603 ± 0.09 b,c | 0.030 ± 0.07 a | |
| 24 | 4.879 ± 0.03 a | 0.186 ± 0.07 c | 1.263 ± 0.02 a | n.d. | 0.334 ± 0.05 a | 0.396 ± 0.04 b,c | 0.003 ± 0.06 a | |
| C | 0 | 0.553 ± 0.13 b | n.d. | 0.964 ± 0.05 a,b | n.d. | 0.335 ± 0.08 a | 0.887 ± 0.09 b | 0.032 ± 0.08 a |
| 4 | 0.339 ± 0.07 b | n.d. | 1.180 ± 0.06 a | n.d. | 0.379 ± 0.06 a,b | 1.252 ± 0.08 b | 0.043 ± 0.03 a | |
| 10 | 0.921 ± 0.06 b | n.d. | 1.212 ± 0.08 b | 1.026 ± 0.07 b | 0.359 ± 0.05 a | 0.986 ± 0.07 b | 0.018 ± 0.08 a | |
| 24 | 3.580 ± 0.01 b | 0.294 ± 0.10 b | 0.533 ± 0.02 b | 0.799 ± 0.10 b | 0.375 ± 0.02 a | 0.633 ± 0.12 b | 0.003 ± 0.07 a | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teleky, B.-E.; Martău, A.G.; Ranga, F.; Chețan, F.; Vodnar, D.C. Exploitation of Lactic Acid Bacteria and Baker’s Yeast as Single or Multiple Starter Cultures of Wheat Flour Dough Enriched with Soy Flour. Biomolecules 2020, 10, 778. https://doi.org/10.3390/biom10050778
Teleky B-E, Martău AG, Ranga F, Chețan F, Vodnar DC. Exploitation of Lactic Acid Bacteria and Baker’s Yeast as Single or Multiple Starter Cultures of Wheat Flour Dough Enriched with Soy Flour. Biomolecules. 2020; 10(5):778. https://doi.org/10.3390/biom10050778
Chicago/Turabian StyleTeleky, Bernadette-Emőke, Adrian Gheorghe Martău, Floricuța Ranga, Felicia Chețan, and Dan C. Vodnar. 2020. "Exploitation of Lactic Acid Bacteria and Baker’s Yeast as Single or Multiple Starter Cultures of Wheat Flour Dough Enriched with Soy Flour" Biomolecules 10, no. 5: 778. https://doi.org/10.3390/biom10050778
APA StyleTeleky, B. -E., Martău, A. G., Ranga, F., Chețan, F., & Vodnar, D. C. (2020). Exploitation of Lactic Acid Bacteria and Baker’s Yeast as Single or Multiple Starter Cultures of Wheat Flour Dough Enriched with Soy Flour. Biomolecules, 10(5), 778. https://doi.org/10.3390/biom10050778