Ampicillin/Sulbactam Treatment Modulates NMDA Receptor NR2B Subunit and Attenuates Neuroinflammation and Alcohol Intake in Male High Alcohol Drinking Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Drinking Protocol
2.2. Brain Dissection
2.3. Western Blot Analyses
2.4. Statistical Analyses
2.4.1. Drinking-Solution Data
2.4.2. Western Blot Data
3. Results
3.1. Effects of AMP/SUL (0, 100 or 200 mg/kg) on Fluid and Food Intake as Well as Body Weight
3.1.1. Effects of AMP/SUL (0 or 100 or 200 mg/kg) on 24 h Ethanol Intake (g/kg/day)
3.1.2. Effects of AMP/SUL (0 or 100 or 200 mg/kg) on 24 h Water Intake (ml/day)
3.1.3. Effects of AMP/SUL (0 or 100 or 200 mg/kg) on Average Body Weight (mL/day)
3.1.4. Effects of AMP/SUL (0 or 100 or 200 mg/kg) on Food Intake (g/day)
3.2. Effects of AMP/SUL (0 or 100 or 200 mg/kg) on the Expression of GLT-1 in the AcbCo and AcbSh of Chronically Ethanol Drinking HAD1 Rats
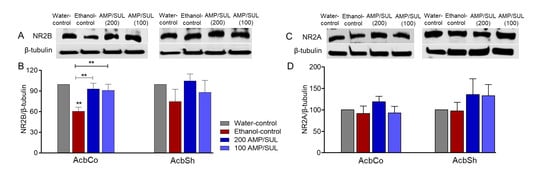
3.3. Effects of AMP/SUL (0 or 100 or 200 mg/kg) on the Expression of NR2B and NR2A in the AcbCo and AcbSh of Chronically Ethanol Drinking HAD1 Rats
3.4. Effects of AMP/SUL (0 or 100 or 200 mg/kg) on the Expression of HMGB1 and RAGE in the AcbCo and AcbSh of Chronically Ethanol Drinking HAD1 Rats
3.5. Effects of AMP/SUL (0 or 100 or 200 mg/kg) on the Expression of TNF-α in the AcbCo and AcbSh of Chronically Ethanol Drinking HAD1 Rats
3.6. Effects of AMP/SUL (0 or 200 mg/kg) on the Expression of mGluR5 in the AcbCo and AcbSh of Chronically Ethanol Drinking HAD1 Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Goodwani, S.; Rao, P.S.; Bell, R.L.; Sari, Y. Amoxicillin and amoxicillin/clavulanate reduce ethanol intake and increase GLT-1 expression as well as AKT phosphorylation in mesocorticolimbic regions. Brain Res. 2015, 1622, 397–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.C.; Yamamoto, B.K.; Hristov, A.M.; Sari, Y. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology 2015, 97, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Z.M.; Rodd, Z.A.; Engleman, E.A.; Bailey, J.A.; Lahiri, D.K.; McBride, W.J. Alcohol drinking and deprivation alter basal extracellular glutamate concentrations and clearance in the mesolimbic system of alcohol-preferring (P) rats. Addict. Biol. 2013, 18, 297–306. [Google Scholar] [CrossRef]
- Griffin , W.C., III; Haun, H.L.; Hazelbaker, C.L.; Ramachandra, V.S.; Becker, H.C. Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology 2014, 39, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Fiorenza, A.M.; Shnitko, T.A.; Sullivan, K.M.; Vemuru, S.R.; Gomez-A, A.; Esaki, J.Y.; Boettiger, C.A.; Da Cunha, C.; Robinson, D.L. Ethanol exposure history and alcoholic reward differentially alter dopamine release in the nucleus accumbens to a reward-predictive cue. Alcohol. Clin. Exp. Res. 2018, 42, 1051–1061. [Google Scholar] [CrossRef]
- Eisenhardt, M.; Leixner, S.; Luján, R.; Spanagel, R.; Bilbao, A. Glutamate receptors within the mesolimbic dopamine system mediate alcohol relapse behavior. J. Neurosci. 2015, 35, 15523–15538. [Google Scholar] [CrossRef] [Green Version]
- Obara, I.; Bell, R.L.; Goulding, S.P.; Reyes, C.M.; Larson, L.A.; Ary, A.W.; Truitt, W.A.; Szumlinski, K.K. Differential effects of chronic ethanol consumption and withdrawal on homer/glutamate receptor expression in subregions of the accumbens and amygdala of P rats. Alcohol. Clin. Exp. Res. 2009, 33, 1924–1934. [Google Scholar] [CrossRef] [Green Version]
- Chaudhri, N.; Sahuque, L.L.; Schairer, W.W.; Janak, P.H. Separable roles of the nucleus accumbens core and shell in context-and cue-induced alcohol-seeking. Neuropsychopharmacology 2010, 35, 783–791. [Google Scholar] [CrossRef]
- Melendez, R.I.; Hicks, M.P.; Cagle, S.S.; Kalivas, P.W. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol. Clin. Exp. Res. 2005, 29, 326–333. [Google Scholar] [CrossRef]
- Rao, P.S.; Goodwani, S.; Bell, R.L.; Wei, Y.; Boddu, S.H.; Sari, Y. Effects of ampicillin, cefazolin and cefoperazone treatments on GLT-1 expressions in the mesocorticolimbic system and ethanol intake in alcohol-preferring rats. Neuroscience 2015, 295, 164–174. [Google Scholar] [CrossRef] [Green Version]
- Sari, Y.; Toalston, J.E.; Rao, P.S.; Bell, R.L. Effects of ceftriaxone on ethanol, nicotine or sucrose intake by alcohol-preferring (P) rats and its association with GLT-1 expression. Neuroscience 2016, 326, 117–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, P.; Saternos, H.; Goodwani, S.; Sari, Y. Effects of ceftriaxone on GLT1 isoforms, xCT and associated signaling pathways in P rats exposed to ethanol. Psychopharmacology 2015, 232, 2333–2342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sari, Y.; Sakai, M.; Weedman, J.M.; Rebec, G.V.; Bell, R.L. Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol Alcohol. 2011, 46, 239–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakami, A.Y.; Sari, Y. β-Lactamase inhibitor, clavulanic acid, attenuates ethanol intake and increases glial glutamate transporters expression in alcohol preferring rats. Neurosci. Lett. 2017, 657, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; John, J.; Langford, D.; Walker, E.; Ward, S.; Rawls, S.M. Clavulanic acid enhances glutamate transporter subtype I (GLT-1) expression and decreases reinforcing efficacy of cocaine in mice. Amino Acids 2016, 48, 689–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cebers, G.; Cebere, A.; Kovács, A.D.; Högberg, H.; Moreira, T.; Liljequist, S. Increased ambient glutamate concentration alters the expression of NMDA receptor subunits in cerebellar granule neurons. Neurochem. Int. 2001, 39, 151–160. [Google Scholar] [CrossRef]
- Kaur, B.; Prakash, A. Ceftriaxone attenuates glutamate-mediated neuro-inflammation and restores BDNF in MPTP model of Parkinson’s disease in rats. Pathophysiology 2017, 24, 71–79. [Google Scholar] [CrossRef]
- Meyer, P.J.; Phillips, T.J. Bivalent effects of MK-801 on ethanol-induced sensitization do not parallel its effects on ethanol-induced tolerance. Behav. Neurosci. 2003, 117, 641. [Google Scholar] [CrossRef] [Green Version]
- Boyce-Rustay, J.M.; Cunningham, C.L. The role of NMDA receptor binding sites in ethanol place conditioning. Behav. Neurosci. 2004, 118, 822. [Google Scholar] [CrossRef]
- Bienkowski, P.; Krzascik, P.; Koros, E.; Kostowski, W.; Scinska, A.; Danysz, W. Effects of a novel uncompetitive NMDA receptor antagonist, MRZ 2/579 on ethanol self-administration and ethanol withdrawal seizures in the rat. Eur. J. Pharmacol. 2001, 413, 81–89. [Google Scholar] [CrossRef]
- Jeanblanc, J.; Coune, F.; Botia, B.; Naassila, M. Brain-derived neurotrophic factor mediates the suppression of alcohol self-administration by memantine. Addict. Biol. 2014, 19, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Hicklin, T.R.; Wu, P.H.; Radcliffe, R.A.; Freund, R.K.; Goebel-Goody, S.M.; Correa, P.R.; Proctor, W.R.; Lombroso, P.J.; Browning, M.D. Alcohol inhibition of the NMDA receptor function, long-term potentiation, and fear learning requires striatal-enriched protein tyrosine phosphatase. Proc. Natl. Acad. Sci. USA 2011, 108, 6650–6655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Carnicella, S.; Phamluong, K.; Jeanblanc, J.; Ronesi, J.A.; Chaudhri, N.; Janak, P.H.; Lovinger, D.M.; Ron, D. Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: Implications for alcohol drinking behavior. J. Neurosci. 2007, 27, 3593–3602. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lanfranco, M.F.; Gibb, S.L.; Yowell, Q.V.; Carnicella, S.; Ron, D. Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse. J. Neurosci. 2010, 30, 10187–10198. [Google Scholar] [CrossRef]
- Floyd, D.W.; Jung, K.-Y.; McCool, B.A. Chronic ethanol ingestion facilitates N-methyl-D-aspartate receptor function and expression in rat lateral/basolateral amygdala neurons. J. Pharmacol. Exp. Ther. 2003, 307, 1020–1029. [Google Scholar] [CrossRef]
- Cozzoli, D.K.; Goulding, S.P.; Zhang, P.W.; Xiao, B.; Hu, J.-H.; Ary, A.W.; Obara, I.; Rahn, A.; Abou-Ziab, H.; Tyrrel, B.; et al. Binge drinking upregulates accumbens mGluR5–Homer2–PI3K signaling: Functional implications for alcoholism. J. Neurosci. 2009, 29, 8655–8668. [Google Scholar] [CrossRef] [Green Version]
- Pian, J.P.; Criado, J.R.; Milner, R.; Ehlers, C.L. N-methyl-D-aspartate receptor subunit expression in adult and adolescent brain following chronic ethanol exposure. Neuroscience 2010, 170, 645–654. [Google Scholar] [CrossRef] [Green Version]
- Goebel, D.J.; Poosch, M.S. NMDA receptor subunit gene expression in the rat brain: A quantitative analysis of endogenous mRNA levels of NR1Com, NR2A, NR2B, NR2C, NR2D and NR3A. Mol. Brain Res. 1999, 69, 164–170. [Google Scholar] [CrossRef]
- Chu, B.; Anantharam, V.; Treistman, S.N. Ethanol inhibition of recombinant heteromeric NMDA channels in the presence and absence of modulators. J. Neurochem. 1995, 65, 140–148. [Google Scholar] [CrossRef]
- Chang, Y.C.; Kim, H.-W.; Rapoport, S.I.; Rao, J.S. Chronic NMDA administration increases neuroinflammatory markers in rat frontal cortex: Cross-talk between excitotoxicity and neuroinflammation. Neurochem. Res. 2008, 33, 2318–2323. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.; Crews, F.T. Glutamate/NMDA excitotoxicity and HMGB1/TLR4 neuroimmune toxicity converge as components of neurodegeneration. Aims Mol. Sci. 2015, 2, 77–100. [Google Scholar] [CrossRef]
- Sil, S.; Ghosh, T.; Ghosh, R. NMDA receptor is involved in neuroinflammation in intracerebroventricular colchicine-injected rats. J. Immunotoxicol. 2016, 13, 474–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodwani, S.; Saternos, H.; Alasmari, F.; Sari, Y. Metabotropic and ionotropic glutamate receptors as potential targets for the treatment of alcohol use disorder. Neurosci. Biobehav. Rev. 2017, 77, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Olive, M.F. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr. Drug Abus. Rev. 2009, 2, 83–98. [Google Scholar] [CrossRef] [Green Version]
- Bäckström, P.; Bachteler, D.; Koch, S.; Hyytiä, P.; Spanagel, R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology 2004, 29, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.-W.; Choi, S.; Lovinger, D.M. Activation of group I mGluRs is necessary for induction of long-term depression at striatal synapses. J. Neurophysiol. 2001, 86, 2405–2412. [Google Scholar] [CrossRef]
- Nandi, A.; Valentine, H.; McCaul, M.; Wong, D. Glutamatergic abnormalities in a rodent model of alcohol abuse. J. Nucl. Med. 2016, 57, 1866a. [Google Scholar]
- de Laat, B.; Weerasekera, A.; Leurquin-Sterk, G.; Gsell, W.; Bormans, G.; Himmelreich, U.; Casteels, C.; Van Laere, K. Effects of alcohol exposure on the glutamatergic system: A combined longitudinal 18F-FPEB and 1H-MRS study in rats. Addict. Biol. 2019, 24, 696–706. [Google Scholar] [CrossRef]
- Byrnes, K.R.; Stoica, B.; Loane, D.J.; Riccio, A.; Davis, M.I.; Faden, A.I. Metabotropic glutamate receptor 5 activation inhibits microglial associated inflammation and neurotoxicity. Glia 2009, 57, 550–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yam Nath Paudel, M.; Shaikh, F.; Chakraborti, A.; Kumari, Y.; Aledo-Serrano, Á.; Aleksovska, K.; Alvim, M.K.M.; Othman, I. HMGB1: A common biomarker and potential target for TBI, neuroinflammation, epilepsy, and cognitive dysfunction. Front. Mol. Neurosci. 2018, 12, 628. [Google Scholar] [CrossRef] [Green Version]
- You, W.-C.; Wang, C.-x.; Pan, Y.-x.; Zhang, X.; Zhou, X.-M.; Zhang, X.-S.; Shi, J.-X.; Zhou, M.-L. Activation of nuclear factor-κB in the brain after experimental subarachnoid hemorrhage and its potential role in delayed brain injury. PLoS ONE 2013, 8, e60290. [Google Scholar] [CrossRef]
- Orio, L.; Alen, F.; Pavón, F.J.; Serrano, A.; García-Bueno, B. Oleoylethanolamide, neuroinflammation and alcohol abuse. Front. Mol. Neurosci. 2018, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- Ureña-Peralta, J.; Alfonso-Loeches, S.; Cuesta-Diaz, C.; García-García, F.; Guerri, C. Deep sequencing and miRNA profiles in alcohol-induced neuroinflammation and the TLR4 response in mice cerebral cortex. Sci. Rep. 2018, 8, 15913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Lizarbe, S.; Pascual, M.; Guerri, C. Critical role of TLR4 response in the activation of microglia induced by ethanol. J. Immunol. 2009, 183, 4733–4744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, L.; He, J.; Hanes, R.N.; Pluzarev, O.; Hong, J.-S.; Crews, F.T. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J. Neuroinflamm. 2008, 5, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, J.Y.; Crews, F.T. Release of neuronal HMGB1 by ethanol through decreased HDAC activity activates brain neuroimmune signaling. PLoS ONE 2014, 9, e87915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antón, M.; Rodríguez-González, A.; Rodríguez-Rojo, I.C.; Pastor, A.; Correas, Á.; Serrano, A.; Ballesta, A.; Alén, F.; Gómez de Heras, R.; de la Torre, R. Increased plasma oleoylethanolamide and palmitoleoylethanolamide levels correlate with inflammatory changes in alcohol binge drinkers: The case of HMGB1 in women. Addict. Biol. 2018, 23, 1242–1250. [Google Scholar] [CrossRef]
- Coleman, L.G.; Zou, J.; Crews, F.T. Microglial-derived miRNA let-7 and HMGB1 contribute to ethanol-induced neurotoxicity via TLR7. J. Neuroinflammation 2017, 14, 22. [Google Scholar] [CrossRef] [Green Version]
- Mayer, S.; Harris, B.R.; Gibson, D.A.; Blanchard, J.A.; Prendergast, M.A.; Holley, R.C.; Littleton, J. Acamprosate, MK-801, and ifenprodil inhibit neurotoxicity and calcium entry induced by ethanol withdrawal in organotypic slice cultures from neonatal rat hippocampus. Alcohol. Clin. Exp. Res. 2002, 26, 1468–1478. [Google Scholar] [CrossRef]
- Foulds, G.; McBride, T.; Knirsch, A.; Rodriguez, W.; Khan, W. Penetration of sulbactam and ampicillin into cerebrospinal fluid of infants and young children with meningitis. Antimicrob. Agents Chemother. 1987, 31, 1703–1705. [Google Scholar] [CrossRef] [Green Version]
- Hammad, A.M.; Alasmari, F.; Althobaiti, Y.S.; Sari, Y. Modulatory effects of Ampicillin/Sulbactam on glial glutamate transporters and metabotropic glutamate receptor 1 as well as reinstatement to cocaine-seeking behavior. Behav. Brain Res. 2017, 332, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Hakami, A.Y.; Alshehri, F.S.; Sari, Y. β-lactams modulate astroglial glutamate transporters and attenuate dependence to CP 55,940, a CB1 receptor agonist, in rat model. Behav. Brain Res. 2019, 359, 709–718. [Google Scholar] [CrossRef]
- Rothstein, J.D.; Patel, S.; Regan, M.R.; Haenggeli, C.; Huang, Y.H.; Bergles, D.E.; Jin, L.; Hoberg, M.D.; Vidensky, S.; Chung, D.S.; et al. β-Lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature 2005, 433, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.L.; Hauser, S.R.; Liang, T.; Sari, Y.; Maldonado-Devincci, A.; Rodd, Z.A. Rat animal models for screening medications to treat alcohol use disorders. Neuropharmacology 2017, 122, 201–243. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.L.; Sable, H.J.; Colombo, G.; Hyytia, P.; Rodd, Z.A.; Lumeng, L. Animal models for medications development targeting alcohol abuse using selectively bred rat lines: Neurobiological and pharmacological validity. Pharmacol. Biochem. Behav. 2012, 103, 119–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBride, W.J.; Rodd, Z.A.; Bell, R.L.; Lumeng, L.; Li, T.K. The alcohol-preferring (P) and high-alcohol-drinking (HAD) rats--animal models of alcoholism. Alcohol 2014, 48, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Murphy, J.M.; Stewart, R.B.; Bell, R.L.; Badia-Elder, N.E.; Carr, L.G.; McBride, W.J.; Lumeng, L.; Li, T.-K. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav. Genet. 2002, 32, 363–388. [Google Scholar] [CrossRef]
- Hammad, A.M.; Sari, Y. Effects of Cocaine Exposure on Astrocytic Glutamate Transporters and Relapse-Like Ethanol-Drinking Behavior in Male Alcohol-Preferring Rats. Alcohol Alcohol. 2020, 55, 254–263. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates in Stereotaxic Coordinates, 6th ed.; Academic Press: San Diego, CA, USA, 2007. [Google Scholar]
- Alasmari, F.; Alexander, L.E.C.; Nelson, J.A.; Schiefer, I.T.; Breen, E.; Drummond, C.A.; Sari, Y. Effects of chronic inhalation of electronic cigarettes containing nicotine on glial glutamate transporters and α-7 nicotinic acetylcholine receptor in female CD-1 mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 77, 1–8. [Google Scholar] [CrossRef]
- Li, J.; Olinger, A.; Dassow, M.; Abel, M. Up-regulation of GABA B receptor mRNA and protein in the hippocampus of cocaine-and lidocaine-kindled rats. Neuroscience 2003, 118, 451–462. [Google Scholar] [CrossRef]
- Raval, A.P.; Dave, K.R.; Mochly-Rosen, D.; Sick, T.J.; Pérez-Pinzón, M.A. εPKC is required for the induction of tolerance by ischemic and NMDA-mediated preconditioning in the organotypic hippocampal slice. J. Neurosci. 2003, 23, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tan, Y. Nerve growth factor augments neuronal responsiveness to noradrenaline in cultured dorsal root ganglion neurons of rats. Neuroscience 2011, 193, 72–79. [Google Scholar] [CrossRef]
- Devoto, V.P.; Bogetti, M.E.; de Plazas, S.F. Developmental and hypoxia-induced cell death share common ultrastructural and biochemical apoptotic features in the central nervous system. Neuroscience 2013, 252, 190–200. [Google Scholar] [CrossRef]
- Alasmari, F.; Bell, R.L.; Rao, P.S.S.; Hammad, A.M.; Sari, Y. Peri-adolescent drinking of ethanol and/or nicotine modulates astroglial glutamate transporters and metabotropic glutamate receptor-1 in female alcohol-preferring rats. Pharmacol. Biochem. Behav. 2018, 170, 44–55. [Google Scholar] [CrossRef] [Green Version]
- Koehler, D.; Shah, Z.A.; Williams, F.E. The GSK3β inhibitor, TDZD-8, rescues cognition in a zebrafish model of okadaic acid-induced Alzheimer’s disease. Neurochem. Int. 2019, 122, 31–37. [Google Scholar] [CrossRef]
- Cui, X.; Li, L.; Hu, Y.-Y.; Ren, S.; Zhang, M.; Li, W.-B. Sulbactam plays neuronal protective effect against brain ischemia via upregulating GLT1 in rats. Mol. Neurobiol. 2015, 51, 1322–1333. [Google Scholar] [CrossRef]
- Alhaddad, H.; Kim, N.T.; Aal-Aaboda, M.; Althobaiti, Y.S.; Leighton, J.; Boddu, S.H.; Wei, Y.; Sari, Y. Effects of MS-153 on chronic ethanol consumption and GLT1 modulation of glutamate levels in male alcohol-preferring rats. Front Behav. Neurosci. 2014, 8, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Althobaiti, Y.S.; Alshehri, F.S.; Hakami, A.Y.; Hammad, A.M.; Sari, Y. Effects of Clavulanic Acid Treatment on Reinstatement to Methamphetamine, Glial Glutamate Transporters, and mGluR 2/3 Expression in P Rats Exposed to Ethanol. J. Mol. Neurosci. 2019, 67, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Wang, W.; Wang, F.; Hu, Z.-L.; Xiao, J.-L.; Yang, S.; Zhang, J.; Peng, X.-Z.; Wang, J.-H.; Chen, J.-G. The stability of NR2B in the nucleus accumbens controls behavioral and synaptic adaptations to chronic stress. Biol. Psychiatry 2013, 74, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.A.; Goto, Y. Chronic stress modulation of prefrontal cortical NMDA receptor expression disrupts limbic structure–prefrontal cortex interaction. Eur. J. Neurosci. 2011, 34, 426–436. [Google Scholar] [CrossRef]
- Qin, L.; Crews, F.T. Chronic ethanol increases systemic TLR3 agonist-induced neuroinflammation and neurodegeneration. J. Neuroinflamm. 2012, 9, 130. [Google Scholar] [CrossRef] [Green Version]
- Montesinos, J.; Gil, A.; Guerri, C. Nalmefene prevents alcohol-induced neuroinflammation and alcohol drinking preference in adolescent female mice: Role of TLR4. Alcohol. Clin. Exp. Res. 2017, 41, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Lippai, D.; Bala, S.; Petrasek, J.; Csak, T.; Levin, I.; Kurt-Jones, E.A.; Szabo, G. Alcohol-induced IL-1β in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. J. Leukoc. Biol. 2013, 94, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Lowe, P.P.; Gyongyosi, B.; Satishchandran, A.; Iracheta-Vellve, A.; Cho, Y.; Ambade, A.; Szabo, G. Reduced gut microbiome protects from alcohol-induced neuroinflammation and alters intestinal and brain inflammasome expression. J. Neuroinflamm. 2018, 15, 298. [Google Scholar] [CrossRef] [PubMed]
- Laird, M.D.; Shields, J.S.; Sukumari-Ramesh, S.; Kimbler, D.E.; Fessler, R.D.; Shakir, B.; Youssef, P.; Yanasak, N.; Vender, J.R.; Dhandapani, K.M. High mobility group box protein-1 promotes cerebral edema after traumatic brain injury via activation of toll-like receptor 4. Glia 2014, 62, 26–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, P.P.; Gyongyosi, B.; Satishchandran, A.; Iracheta-Vellve, A.; Ambade, A.; Kodys, K.; Catalano, D.; Ward, D.V.; Szabo, G. Alcohol-related changes in the intestinal microbiome influence neutrophil infiltration, inflammation and steatosis in early alcoholic hepatitis in mice. PLoS ONE 2017, 12, e0174554. [Google Scholar]
- Zhang, Z.-y.; Sun, B.-l.; Liu, J.-k.; Yang, M.-f.; Li, D.-w.; Fang, J.; Zhang, S.; Yuan, Q.-l.; Huang, S.-l. Activation of mGluR5 attenuates microglial activation and neuronal apoptosis in early brain injury after experimental subarachnoid hemorrhage in rats. Neurochem. Res. 2015, 40, 1121–1132. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alasmari, F.; Alhaddad, H.; Wong, W.; Bell, R.L.; Sari, Y. Ampicillin/Sulbactam Treatment Modulates NMDA Receptor NR2B Subunit and Attenuates Neuroinflammation and Alcohol Intake in Male High Alcohol Drinking Rats. Biomolecules 2020, 10, 1030. https://doi.org/10.3390/biom10071030
Alasmari F, Alhaddad H, Wong W, Bell RL, Sari Y. Ampicillin/Sulbactam Treatment Modulates NMDA Receptor NR2B Subunit and Attenuates Neuroinflammation and Alcohol Intake in Male High Alcohol Drinking Rats. Biomolecules. 2020; 10(7):1030. https://doi.org/10.3390/biom10071030
Chicago/Turabian StyleAlasmari, Fawaz, Hasan Alhaddad, Woonyen Wong, Richard L. Bell, and Youssef Sari. 2020. "Ampicillin/Sulbactam Treatment Modulates NMDA Receptor NR2B Subunit and Attenuates Neuroinflammation and Alcohol Intake in Male High Alcohol Drinking Rats" Biomolecules 10, no. 7: 1030. https://doi.org/10.3390/biom10071030
APA StyleAlasmari, F., Alhaddad, H., Wong, W., Bell, R. L., & Sari, Y. (2020). Ampicillin/Sulbactam Treatment Modulates NMDA Receptor NR2B Subunit and Attenuates Neuroinflammation and Alcohol Intake in Male High Alcohol Drinking Rats. Biomolecules, 10(7), 1030. https://doi.org/10.3390/biom10071030