The Interaction of Bovine β-Lactoglobulin with Caffeic Acid: From Binding Mechanisms to Functional Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. β-Lactoglobulin Cross-Linking
2.3. Heat Treatment
2.4. Quenching Experiments
2.5. Molecular Modeling Investigations
2.6. β-LG-Caffeic Acid Complexation
2.7. Antioxidant Activity
2.8. α-Glucosidase Inhibition
2.9. α-Amylase Inhibition
2.10. Lipase Inhibition
2.11. Statistical Analysis
3. Results and Discussion
3.1. Binding Mechanisms
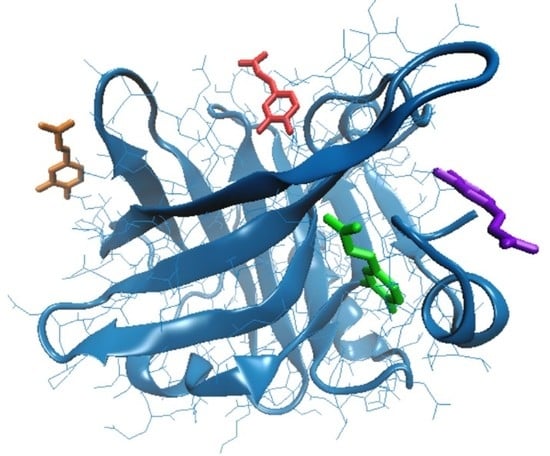
3.2. In Silico Investigation on Caffeic Acid Binding by β-LG
3.3. Effect of Complexation on Antioxidant Activity
3.4. Impact of Complexation on Enzyme Inhibitory Effect
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Genaro-Mattos, T.C.; Maurício, Â.Q.; Rettori, D.; Alonso, A.; Hermes-Lima, M. Antioxidant activity of caffeic acid against iron-induced free radical generation-A chemical approach. PLoS ONE 2015, 10, e0129963. [Google Scholar]
- Lin, Y.; Yan, Y. Byosinthesis of caffeic acid in Escherichia coli using its endogenous hydroxylase complex. Microb. Cell Fact. 2012, 11, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, J.L.; Araújo, R.G.; Prather, K.L.J.; Kluskens, L.D.; Rodrigues, L.R. Heterologous production of caffeic acid from tyrosine in Escherichia coli. Enzyme Microb. Technol. 2015, 71, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Lin, Y.; Yan, Y. Caffeic acid production enhancement by engineering a phenylalanine over-producing Escherichia coli strain. Biotechnol. Bioeng. 2013, 110, 3188–3196. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Oliveira, C.; Borges, F. Caffeic acid derivatives, analogs and applications: A patent review (2009–2013). Expert Opin. Ther. Pat. 2014, 24, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Kilani-Jaziri, S.; Mokdad-Bzeouich, I.; Krifa, M.; Nasr, N.; Ghedira, K.; ChekirGhedira, L. Immunomodulatory and cellular anti-oxidant activities of caffeic, ferulic, and p-coumaric phenolic acids: A structure-activity relationship study. Drug Chem. Toxicol. 2017, 40, 416–424. [Google Scholar] [CrossRef]
- Agunloye, O.M.; Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Akindahunsi, A.A.; Oyagbemi, A.A.; Omobowale, T.O.; Ajibade, T.O.; Adedapo, A.A. Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: Mechanistic role of angiotensin converting enzyme, cholinesterase and arginase activities in cyclosporine induced hypertensive rats. Biomed. Pharmacother. 2019, 109, 450–458. [Google Scholar] [CrossRef]
- Nagaoka, T.; Banskota, A.H.; Tezuka, Y.; Saiki, I.; Kadota, S. Selective antiproliferative activity of caffeic acid phenethyl ester analogues on highly liver-Metastatic murine colon 26-L5 carcinoma cell line. Bioorg. Med. Chem. 2002, 10, 3351–3359. [Google Scholar] [CrossRef]
- Bispo, V.S.; Dantas, L.S.; Chaves Filho, A.B.; Pinto, I.F.D.; Silva, R.P.D.A.; Otsuka, F.A.M.; Santos, R.B.; Santos, A.C.; Trindade, D.J.; Matos, H.R. Reduction of the DNA damages, hepatoprotective effect and antioxidant potential of the coconut water, ascorbic and caffeic acids in oxidative stress mediated by ethanol. Anais Acad. Brasil. Ciênc. 2017, 89, 1095–1099. [Google Scholar] [CrossRef]
- Gu, W.; Yang, Y.; Zhang, C.; Zhang, Y.; Chen, L.; Shen, J.; Li, G.; Li, Z.; Li, Y.; Dong, H. Caffeic acid attenuates the angiogenic function of hepatocellular carcinoma cells via reduction in JNK-1-mediated HIF-1α stabilization in hypoxia. RSC Adv. 2016, 6, 82774–82782. [Google Scholar] [CrossRef]
- Pessato, T.B.; de Carvalho, N.C.; de Figueiredo, D.; Colomeu, T.C.; Fernandes, L.G.R.; Netto, F.M.; Zollner, R.D.L. Complexation of whey protein with caffeic acid or (−)-epigallocatechin-3-gallate as a strategy to induce oral tolerance to whey allergenic proteins. Int. Immunopharmacol. 2019, 68, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Corrochano, A.R.; Buckin, V.; Kelly, P.M.; Giblin, L. Invited review: Whey proteins as antioxidants and promoters of cellular antioxidant pathways. J. Dairy Sci. 2018, 101, 4747–4761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafaei, Z.; Ghalandari, B.; Vaseghi, A.; Divsalar, A.; Haertl, T.; Saboury, A.A.; Sawyer, L. β-Lactoglobulin: An efficient nanocarrier for advanced delivery systems. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1685–1692. [Google Scholar] [CrossRef] [PubMed]
- Taulier, N.; Chalikian, T.V. Characterization of pH-induced transitions of β-Lactoglobulin: Ultrasonic, densimetric, and spectroscopic studies. J. Mol. Biol. 2001, 314, 873–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Shabib, N.A.; Khan, J.M.; Malik, A.; Alsenaidy, M.A.; Rehman, M.T.; AlAjmi, M.F.; Alsenaidy, A.M.; Husain, F.M.; HasanKhan, R. Molecular insight into binding behavior of polyphenol (rutin) with β-lactoglobulin: Spectroscopic, molecular docking and MD simulation studies. J. Mol. Liq. 2018, 269, 511–520. [Google Scholar] [CrossRef]
- Qin, B.Y.; Bewley, M.C.; Creamer, L.K.; Baker, H.M.; Baker, E.N.; Jameson, G.B. Structural basis of the Tanford transition of bovine beta-lactoglobulin. Biochemistry 1998, 37, 14014–14023. [Google Scholar] [CrossRef]
- Domínguez-Ramírez, L.; Del Moral-Ramírez, E.; Cortes-Hernández, P.; García-Garibay, M.; Jiménez-Guzmán, J. β-Lactoglobulin’s conformational requirements for ligand binding at the calyx and the dimer interphase: A flexible docking study. PLoS ONE 2013, 8, e79530. [Google Scholar] [CrossRef] [Green Version]
- Tai, C.S.; Chen, Y.Y.; Chen, W.L. β-Lactoglobulin influences human immunity and promotes cell proliferation. BioMed Res. Int. 2016, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.-L. Sepsis and septic shock. Nat. Rev. Dis. Primers 2016, 2, 1–47. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Bai, Y.; Gao, J.; Li, X.; Chen, H. Effects of high hydrostatic pressure on the structure and potential allergenicity of the major allergen bovine β-lactoglobulin. Food Chem. 2017, 219, 290–296. [Google Scholar] [CrossRef]
- Buszewski, B.; Rodzik, A.; Railean-Plugaru, V.; Sprynskyy, M. A study of zinc ions immobilization by β-lactoglobulin. Colloids Surf. Physicochem. Eng. Asp. 2020, 591, 1244432020. [Google Scholar] [CrossRef]
- Grossmann, L.; Wefers, D.; Bunzel, M.; Weiss, J.; Zeeb, B. Accessibility of transglutaminase to induce protein crosslinking in gelled food matrices - Influence of network structure. LWT 2018, 75, 271–278. [Google Scholar] [CrossRef]
- Aprodu, I.; Ursache, F.M.; Turturica, M.; Râpeanu, G.; Stanciuc, N. Thermal stability of the complex formed between carotenoids from sea buckthorn (Hippophae rhamnoides L.) and bovine β-lactoglobulin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 173, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Oancea, A.M.; Hasan, M.; Vasile, A.M.; Barbu, V.; Enachi, E.; Bahrim, G.; Răpeanu, G.; Silvi, S.; Stănciuc, N. Functional evaluation of microencapsulated anthocyanins from sour cherries skins extract in whey proteins isolate. LWT Food Sci. Technol. 2018, 95, 129–134. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford Method for Protein Quantitation. In The Protein Protocols Handbook, 2nd ed.; Walker, J.M., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2002; pp. 15–23. [Google Scholar]
- Dumitrașcu, L.; Stănciuc, N.; Aprodu, I. New insights into xanthine oxidase behavior upon heating using spectroscopy and in silico approach. Int. J. Biol. Macromol. 2016, 88, 306–312. [Google Scholar] [CrossRef]
- Loch, J.I.; Bonarek, P.; Polit, A.; Riès, D.; Dziedzicka-Wasylewska, M.; Lewiński, K. Binding of 18-carbon unsaturated fatty acids to bovine β-lactoglobulin—structural and thermodynamic studies. Int. J. Biol. Macromol. 2013, 7, 226–231. [Google Scholar] [CrossRef]
- Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef] [Green Version]
- Schneidman-Duhovny, D.; Inbar, Y.; Nussinov, R.; Wolfso, H.J. PatchDock and SymmDock: Servers for rigid and symmetric docking. Nucleic Acids Res. 2005, 33, W363–W367. [Google Scholar] [CrossRef] [Green Version]
- Krissinel, E. Crystal contacts as nature’s docking solutions. J. Comput. Chem. 2010, 31, 133–143. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Kandi, S.; Charles, A.L. Statistical comparative study between the conventional DPPH spectrophotometric and dropping DPPH· analytical method without spectrophotometer: Evaluation for the advancement of antioxidant activity analysis. Food Chem. 2019, 287, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Manabe, Y.; Sugawara, T.; Paul, N.A.; Zhao, J. Identification and biological activities of carotenoids from the freshwater alga Oedogonium intermedium. Food Chem. 2018, 242, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Costamagna, M.S.; Zampini, I.C.; Alberto, M.R.; Cuello, S.; Torres, S.; Pérez, J.; Quispe, C.; Schmeda-Hirschmann, G.; Isla, M.I. Polyphenols rich fraction from Geoffroea decorticans fruits flour affects key enzymes involved in metabolic syndrome, oxidative stress and inflammatory process. Food Chem. 2016, 190, 392–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eissa, A.S.; Puhl, C.; Kadla, J.F.; Khan, S.A. Enzymatic cross-linking of β-lactoglobulin: Conformational properties using FTIR spectroscopy. Biomacromolecules 2006, 7, 5010–5017. [Google Scholar] [CrossRef]
- Loveday, S.M. β-Lactoglobulin heat denaturation: A critical assessment of kinetic modelling. Int. Dairy J. 2016, 52, 92–100. [Google Scholar] [CrossRef]
- Anema, S.G.; McKenna, A.B. Reaction kinetics of thermal denaturation of whey proteins in heated reconstituted whole milk. J. Agric. Food Chem. 1996, 44, 422–428. [Google Scholar] [CrossRef]
- Iametti, S.; Cairoli, S.; Gregori, B.; de Bonomi, F. Modifications of high-order structures upon heating of β-lactoglobulin: Dependence on the protein concentration. J. Agric. Food Chem. 1995, 43, 53–58. [Google Scholar] [CrossRef]
- Lakowicz, J. Principles of Fluorescence Spectroscopy; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999. [Google Scholar]
- Stojadinovic, M.; Radosavljevic, J.; Ognjenovic, J.; Vesic, J.; Prodic, I.; Stanic-Vucinic, D.; Cirkovic Velickovic, T. Binding affinity between dietary polyphenols and β-lactoglobulin negatively correlates with the protein susceptibility to digestion and total antioxidant activity of complexes formed. Food Chem. 2013, 136, 1263–1271. [Google Scholar] [CrossRef]
- Klajnert, B.; Stanisławska, L.; Bryszewska, M.; Pałecz, B. Interactions between PAMAM dendrimers and bovine serum albumin. BBA Proteins Proteom. 2003, 1648, 115–126. [Google Scholar] [CrossRef]
- Gholami, S.; Bordbar, A.K. Exploring binding properties of naringenin with bovine β-lactoglobulin: A fluorescence, molecular docking and molecular dynamics simulation study. Biophys. Chem. 2014, 187–188, 33–42. [Google Scholar] [CrossRef]
- Arroyo-Maya, I.J.; Campos-Terán, J.; Hernández-Arana, A.; McClements, D.J. Characterization of flavonoid-protein interactions using fluorescence spectroscopy: Binding of pelargonidin to dairy proteins. Food Chem. 2016, 213, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef] [PubMed]
- Stănciuc, N.; Aprodu, I.; Râpeanu, G.; Bahrim, G. Fluorescence spectroscopy and molecular modeling investigations on the thermally induced structural changes of bovine β-lactoglobulin. Innov. Food Sci. Emerg. Technol. 2012, 15, 50–56. [Google Scholar] [CrossRef]
- Loch, J.; Polit, A.; Górecki, A.; Bonarek, P.; Kurpiewska, K.; Dziedzicka-Wasylewska, M.; Lewiński, K. Two modes of fatty acid binding to bovine β-lactoglobulin—Crystallographic and spectroscopic studies. J. Mol. Recog. 2011, 24, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B.J.; Bell, E.; Brancaleon, L.A. Tryptophan rotamer located in a polar environment probes pH-dependent conformational changes in bovine β-lactoglobulin A. J. Phys. Chem. B 2007, 111, 2610–2620. [Google Scholar] [CrossRef] [PubMed]
- Krissinel, E.; Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007, 372, 774–797. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Obesity and Overweight. 2017. Available online: http://www.who.int/news-rom/fact-sheets/detail/obesity-and-overweight (accessed on 4 July 2020).
- Kwon, Y.I.; Apostolidis, E.; Shetty, K. In vitro studies of eggplant (Solanum melongena) phenolics as inhibitors of key enzymes relevant for type 2 diabetes and hypertension. Biores. Technol. 2007, 99, 2981–2988. [Google Scholar] [CrossRef]
- Sosnowska, D.; Podsędek, A.; Redzynia, M.; Kucharska, A.Z. Inhibitory effect of black chokeberry fruit polyphenols on pancreatic lipase—Searching for most active inhibitors. J. Funct. Food 2018, 49, 196–204. [Google Scholar] [CrossRef]
- Garza, A.L.; Milagro, F.I.; Boque, N.; Campion, J.; Martinez, J.A. Natural inhibitors of pancreatic lipase as new players in obesity treatment. Planta Med. 2011, 77, 773–785. [Google Scholar] [CrossRef] [Green Version]



| n-β-LG | β-LGTgase | n-β-LG | β-LGTgage | n-β-LG | β-LGTgase | |
|---|---|---|---|---|---|---|
| T (°C) | KSV (106 Mol-1) | Kb (106 Mol-1) | n | |||
| 25 | 25.46 ± 0.72 a1 | 35.33 ± 1.41 a | 0.72 ± 0.03 c | 0.52 ± 0.03 b | 0.56 ± 0.02 a | 0.65 ± 0.07 b |
| 75 | 21.89 ± 0.42 b | 27.50 ± 1.53 b | 0.83 ± 0.10 b | 0.77 ± 0.06 a,b | 0.63 ± 0.06 a | 0.81 ± 0.08 b |
| 85 | 20.24 ± 0.10 c | 14.50 ± 0.70 c | 0.89 ± 0.01 a,b | 0.85 ± 0.03 a,b | 0.69 ± 0.01 a | 0.89 ± 0.04 b |
| 95 | 17.34 ± 0.54 d | 12.50 ± 1.12 c | 0.96 ± 0.01 a | 1.04 ± 0.21 a | 0.66 ± 0.10 a | 1.32 ± 0.28 a |
| T(K) | ΔH (J/Mol) | ΔS (J/Mol·K) | ΔG (J/Mol) | |
|---|---|---|---|---|
| n-β-LG | 298 | −418.72 ± 11.58 | −14.87 ± 1.36 | 4012.54 ± 21.34 |
| 348 | 4756.04 ± 14.32 | |||
| 358 | 4904.74 ± 12.21 | |||
| 368 | 5053.44 ± 14.58 | |||
| Tgase-β-LG | 298 | −983.76 ± 23.45 | −16.42 ± 2.01 | 3915.95 ± 16.78 |
| 348 | 4738.05 ± 19.09 | |||
| 358 | 4902.47 ± 18.74 | |||
| 368 | 5066.89 ± 14.53 |
| Descriptors | Temperature, °C | |||
|---|---|---|---|---|
| 25 | 75 | 85 | 95 | |
| Total protein surface, Å2 | 8546.19 ± 88.69 | 8585.87 ± 170.85 | 8212.59 ± 130.76 | 8167.92 ± 125.47 |
| Hydrophobic protein surface, Å2 | 4953.24 ± 67.28 | 4760.91 ± 134.34 | 4597.87 ± 120.93 | 4605.74 ± 94.34 |
| βLG –CA interface area, Å2 | 216.2 | 210.9 | 178.0 | 129.3 |
| Particularities of the binding site | ||||
| Amino acids interacting with CA | Tyr20, Tyr42, Val43, Glu44, Gln59, Cys66, Gln68, Leu156, Glu157, Glu158, Gln159, Cys160, His161 | Gln35, Arg40, Tyr42, Trp61, Cys66, Glu158, Gln159, Cys160, His161, Ile162 | Leu31, Pro38, Leu39, Val41, Leu58, Lys60, Asn90, Met107, Asn109, Gln115, Ser116 | Glu74, Lys75, Thr76, Lys83, Ala86, Leu87, |
| Amino acids involved in HB with CA (HB length) | Leu156 (2.1Å) | Gln35 (3.51Å), Gln159 (2.92Å) | Lys60 (2.79Å) | Thr76 (3.01Å), Lys83 (3.24Å) |
| Total cavity surface, Ų | 293.69 | 417.47 | 239.55 | 402.18 |
| Total cavity depth, Å | 9.42 | 9.37 | 4.88 | 11.69 |
| βLG–CA binding energy, kcal/mol | −23.23 | −24.75 | −18.33 | −19.72 |
| ΔGf, kcal/mol | −151.9 | −158.6 | −158.3 | −166.0 |
| ΔGint, kcal/mol | 2.6 | 1.9 | 1.2 | 0.8 |
| TΔSdiss, kcal/mol | 2.8 | 2.8 | 2.8 | 2.8 |
| ΔGdiss, kcal/mol | −5.4 | −4.7 | −4.0 | −4.6 |
| Enzymes | Native β-LG | Complexation Ratio 1:1 | Complexation Ratio 1:2 | ||
|---|---|---|---|---|---|
| Native β-LG-CA | Cross-linked β-LG-CA | Native β-LG-CA | Cross-linked β-LG-CA | ||
| Enzymes related to carbohydrate metabolism | |||||
| α-Glucosidase | 57.13 ± 1.31 a1 | 47.12 ± 2.60 b | 44.95 ± 4.28 b | 35.36 ± 3.52 c | 36.22 ± 3.38 c |
| α-Amylase | 25.63 ± 0.80 b | 44.27 ± 0.33 a | 40.38 ± 3.62 a | 11.89 ± 0.24 c | 15.02 ± 1.21 c |
| Enzymes related to fat metabolism | |||||
| Lipase | 23.58 ± 2.14 a | 8.74 ± 0.93 c | 14.23 ± 2.31 b | 2.21 ± 0.24 d | 4.42 ± 0.53 d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stănciuc, N.; Râpeanu, G.; Bahrim, G.E.; Aprodu, I. The Interaction of Bovine β-Lactoglobulin with Caffeic Acid: From Binding Mechanisms to Functional Complexes. Biomolecules 2020, 10, 1096. https://doi.org/10.3390/biom10081096
Stănciuc N, Râpeanu G, Bahrim GE, Aprodu I. The Interaction of Bovine β-Lactoglobulin with Caffeic Acid: From Binding Mechanisms to Functional Complexes. Biomolecules. 2020; 10(8):1096. https://doi.org/10.3390/biom10081096
Chicago/Turabian StyleStănciuc, Nicoleta, Gabriela Râpeanu, Gabriela Elena Bahrim, and Iuliana Aprodu. 2020. "The Interaction of Bovine β-Lactoglobulin with Caffeic Acid: From Binding Mechanisms to Functional Complexes" Biomolecules 10, no. 8: 1096. https://doi.org/10.3390/biom10081096
APA StyleStănciuc, N., Râpeanu, G., Bahrim, G. E., & Aprodu, I. (2020). The Interaction of Bovine β-Lactoglobulin with Caffeic Acid: From Binding Mechanisms to Functional Complexes. Biomolecules, 10(8), 1096. https://doi.org/10.3390/biom10081096