Effect of Methionine Diet on Time-Related Metabolic and Histopathological Changes of Rat Hippocampus in the Model of Global Brain Ischemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Induction of Ischemia-Reperfusion Injury (IRI)
2.2. Induction of Mild Hyperhomocysteinemia (hHcy) by Met Enriched Diet
2.3. Experimental Groups of Animals
- (1)
- control (naïve) animals (C, n = 5).
- (2)
- naïve animals that underwent 15 min ischemia and 3 days of reperfusion (IR-3d, n = 5).
- (3)
- naïve animals that underwent 15 min ischemia and 7 days of reperfusion (IR-7d, n = 5).
- (4)
- animals after 28 days with Met diet without ischemic insult–Met control (Met-C, n = 5).
- (5)
- animals after 28 days with Met diet that underwent 15 min ischemia and 3 days of reperfusion (Met-IR-3d, n = 5).
- (6)
- animals after 28 days with Met diet that underwent 15 min ischemia and 7 days of reperfusion (Met-IR-7d, n = 5).
2.4. FluoroJade-C Staining
2.5. Fluorescent Immunohistochemistry
2.6. In Vivo Magnetic Resonance Examination
2.7. Image Analysis
2.8. Statistical Analysis
3. Results
3.1. Determination of Plasma Homocysteine Level
3.2. Histo-Morphological Changes in the Rat CA1 of Hippocampus
3.2.1. FluoroJade-C (FJC) Staining
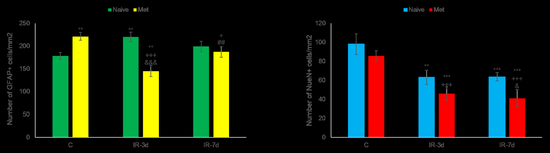
3.2.2. NeuN and Glial Fibrillary Acidic Protein (GFAP) Measurements
3.3. 1H Magnetic Resonance Spectroscopy (MRS) Analysis and Volumetry
4. Discussion
4.1. Histo-Morphological Changes in the Hippocampus after Met Diet Induced Hyperhomocysteinemia (hHcy) and Effect of IRI
4.2. In Vivo Metabolic Changes in the Brain after Met Diet Induced hHcy and the Effect of Ischemia-Reperfusion Insult (IRI)
4.3. Volumetric Changes in the Brain after Met Diet-Induced hHcy
5. Conclusions
6. Limitations of Study
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| CA1 | Cornu ammonis 1 |
| FJC | FluoroJade-C |
| GFAP | Glial fibrillary acidic protein |
| Hcy | Homocysteine |
| hHcy | Hyperhomocysteinemia |
| 1H MRS | Proton magnetic resonance spectroscopy |
| hMet | Hypermethioninemia |
| Met | Methionine |
| mIns | Myo-Inositol |
| MRI | Magnetic resonance imaging |
| NeuN | Neural nuclei |
| tCho | Total choline |
| tCr | Total creatinine |
| tNAA | Total N-ademosyl-aspartate |
References
- Xu, Y.; Tian, Y.; Wei, H.J.; Dong, J.F.; Zhang, J.N. Methionine diet-induced hyperhomocysteinemia accelerates cerebral aneurysm formation in rats. Neurosci. Lett. 2011, 494, 139–144. [Google Scholar] [CrossRef]
- Berti, V.; Murray, J.; Davies, M.; Spector, N.; Tsui, W.H.; Li, Y.; Williams, S.; Pirraglia, E.; Vallabhajosula, S.; McHugh, P.; et al. Nutrient patterns and brain biomarkers of Alzheimer’s disease in cognitively normal individuals. J. Nutr. Health Aging 2015, 19, 413–423. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Ma, S.; Wang, T.; Zhao, C.; Li, Y.; Yin, J.; Liu, C.; Gao, C.; Sun, L.; Yue, W.; et al. A novel rat model of heart failure induced by high methionine diet showing evidence of association between hyperhomocysteinemia and activation of NF-kappaB. Am. J. Transl. Res. 2016, 8, 117–124. [Google Scholar]
- Esse, R.; Barroso, M.; de Almeida, I.T.; Castro, R. The Contribution of Homocysteine Metabolism Disruption to Endothelial Dysfunction: State-of-the-Art. Int. J. Mol. Sci. 2019, 20, 867. [Google Scholar] [CrossRef] [Green Version]
- Lehotsky, J.; Kovalska, M.; Tomascova, A.; Kalenska, D.; Baranovicova, E.; Kaplan, P. Ischemic brain injury in hyperhomocysteinemic conditions and the development of Alzheimer’s disease. In Brain Ischemia: Alzheimer’s Disease Mechanisms, 1st ed.; Pluta, R., Ed.; Nova Science Pub Inc.: New York, NY, USA, 2019; pp. 115–156. [Google Scholar]
- Soares, M.S.; de Mattos, B.D.; de Souza, A.Á.; Spohr, L.; Tavares, R.G.; Siebert, C.; de Souza, M.D.; Wyse, A.T.; Carvalho, F.B.; Rahmeier, F.; et al. Hypermethioninemia induces memory deficits and morphological changes in hippocampus of young rats: Implications on pathogenesis. Amino Acids 2020, 52, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Tarrago, L.; Péterfi, Z.; Lee, B.C.; Michel, T.; Gladyshev, V.N. Monitoring methionine sulfoxide with stereospecific mechanism-based fluorescent sensors. Nat. Chem. Biol. 2015, 11, 332–338. [Google Scholar] [CrossRef]
- Suzuki, S.; Kodera, Y.; Saito, T.; Fujimoto, K.; Momozono, A.; Hayashi, A.; Kamata, Y.; Shichiri, M. Methionine sulfoxides in serum proteins as potential clinical biomarkers of oxidative stress. Sci. Rep. 2016, 6, 38299–38309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanello, F.M.; Chiarani, F.; Kurek, A.G.; Wannmacher, C.M.D.; Wajner, M.; Wyse, A.T. Methionine alters Na+,K+-ATPase activity, lipid peroxidation and nonenzymatic antioxidant defenses in rat hippocampus. Int. J. Dev. Neurosci. 2005, 23, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, F.M.; Matté, C.; Scherer, E.B.; Wannmacher, C.M.D.; Wajner, M.; Wyse, A.T.S. Chemically induced model of hypermethioninemia in rats. J. Neurosci. Methods 2007, 160, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.S.P.; Viau, C.M.; Saffi, J.; Costa, M.Z.; da Silva, T.M.; Oliveira, P.S.; Azambuja, J.H.; Barschak, A.G.; Braganhol, E.; Wyse, A.T.; et al. Acute administration of methionine and/or methionine sulfoxide impairs redox status and induces apoptosis in rat cerebral cortex. Metab. Brain Dis. 2007, 32, 1693–1703. [Google Scholar] [CrossRef]
- Gour, N.; Kanth, P.C.; Koshti, B.; Kshtriya, V.; Shah, D.; Patel, S.; Agrawal-Rajput, R.; Pandey, M.K. Amyloid-like Structures Formed by Single Amino Acid Self-Assemblies of Cysteine and Methionine. ACS Chem. Neurosci. 2019, 10, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, A.; Chan, G.K.L.; Boatz, J.C.; Li, N.J.; Inoue, A.P.; Wong, J.C.; van der Wel, P.C.A.; Cavigiolio, G. Methionine oxidized apolipoprotein A-I at the crossroads of HDL biogenesis and amyloid formation. FASEB J. 2018, 32, 3149–3165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bugnicourt, J.M.; Godefroy, O.; Chillon, J.M.; Choukroun, G.; Massy, Z.A. Cognitive Disorders and Dementia in CKD: The Neglected Kidney-Brain Axis. JASN 2013, 24, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, A.H.; Yeo, N.E.; Weekman, E.M.; Wilcock, D.M. Homocysteine, hyperhomocysteinemia and vascular contributions to cognitive impairment and dementia (VCID). Biochim. Biophys. Acta 2016, 1862, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Weekman, E.M.; Sudduth, T.L.; Price, B.R.; Woolums, A.E.; Hawthorne, D.; Seaks, C.E.; Wilcock, D.M. Time course of neuropathological events in hyperhomocysteinemic amyloid depositing mice reveals early neuroinflammatory changes that precede amyloid changes and cerebrovascular events. J. Neuroinflamm. 2019, 16, 284–295. [Google Scholar] [CrossRef]
- Charidimou, A.; Werring, D.J. Cerebral microbleeds: Detection, mechanisms and clinical challenges. Future Neurol. 2011, 6, 587–611. [Google Scholar] [CrossRef] [Green Version]
- Low, A.; Mak, E.; Rowe, J.B.; Markus, H.S.; O’Brien, J.T. Inflammation and cerebral small vessel disease: A systematic review. Ageing Res. Rev. 2019, 53, 100916–100944. [Google Scholar] [CrossRef]
- Rizk, T.; Turtzo, L.C.; Cota, M.; Van Der Merwe, A.J.; Latour, L.; Whiting, M.D.; Chan, L. Traumatic microbleeds persist for up to five years following traumatic brain injury despite resolution of other acute findings on MRI. Brain Injury 2020, 34, 773–781. [Google Scholar] [CrossRef]
- Kovalska, M.; Hnilicova, P.; Kalenska, D.; Tothova, B.; Adamkov, M.; Lehotsky, J. Effect of Methionine Diet on Metabolic and Histopathological Changes of Rat Hippocampus. Int. J. Mol. Sci. 2019, 20, 6234. [Google Scholar] [CrossRef] [Green Version]
- Kovalska, M.; Kovalska, L.; Tothova, B.; Mahmood, S.; Adamkov, M.; Lehotsky, J. Combination of hyperhomocysteinemia and ischemic tolerance in experimental model of global ischemia in rats. J. Physiol. Pharmacol. 2015, 66, 887–897. [Google Scholar]
- Kovalska, M.; Tothova, B.; Kovalska, L.; Tatarkova, Z.; Kalenska, D.; Tomascova, A.; Adamkov, M.; Lehotsky, J. Association of Induced Hyperhomocysteinemia with Alzheimer’s Disease-Like Neurodegeneration in Rat Cortical Neurons After Global Ischemia-Reperfusion Injury. Neurochem. Res. 2018, 43, 1766–1778. [Google Scholar] [CrossRef] [PubMed]
- Kovalska, M.; Tothova, B.; Kalenska, D.; Tomascova, A.; Kovalska, L.; Adamkov, M.; Lehotsky, J. Association of induced hyperhomocysteinemia with neurodegeneration in rat entorhinal cortex-hippocampal system after global brain ischemia: A progression of Alzheimer’s disease-like pathological features? Act. Nerv. Super. Rediviva 2019, 61, 31–38. [Google Scholar]
- Tóthová, B.; Kovalská, M.; Kalenská, D.; Tomašcová, A.; Lehotský, J. Histone Hyperacetylation as a Response to Global Brain Ischemia Associated with Hyperhomocysteinemia in Rats. Int. J. Mol. Sci. 2018, 19, 3147. [Google Scholar] [CrossRef] [Green Version]
- Pulsinelli, W.A.; Brierley, J.B.; Plum, F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann. Neurol. 1982, 11, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: London, UK, 2007; p. 456. [Google Scholar]
- Ding, G.L.; Chopp, M.; Li, L.; Zhang, L.; Zhang, Z.G.; Li, Q.J.; Jian, Q. Magnetic Resonance Imaging of Stroke in the Rat. Bo Pu Xue Za Zhi 2014, 31, 116–132. [Google Scholar] [PubMed]
- Pluta, R.; Ułamek-Kozioł, M.; Kocki, J.; Bogucki, J.; Januszewski, S.; Bogucka-Kocka, A.; Czuczwar, S.J. Expression of the Tau Protein and Amyloid Protein Precursor Processing Genes in the CA3 Area of the Hippocampus in the Ischemic Model of Alzheimer’s Disease in the Rat. Mol. Neurobiol. 2020, 57, 1281–1290. [Google Scholar] [CrossRef] [Green Version]
- Newrezella, D.; Pahlavan, P.S.; Krüger, C.; Boehm, C.; Sorgenfrei, O.; Schröck, H.; Eisenhardt, G.; Bischoff, N.; Vogt, G.; Wafzig, O.; et al. The functional genome of CA1 and CA3 neurons under native conditions and in response to ischemia. BMC Genom. 2007, 8, 370. [Google Scholar] [CrossRef] [Green Version]
- Stetler, R.A.; Leak, R.K.; Gan, Y.; Li, P.; Zhang, F.; Hu, X.; Jing, Z.; Chen, J.; Zigmond, M.J.; Gao, Y. Preconditioning provides neuroprotection in models of CNS disease: Paradigms and clinical significance. Prog Neurobiol. 2014, 114, 58–83. [Google Scholar] [CrossRef] [Green Version]
- Lehotský, J.; Tothová, B.; Kovalská, M.; Dobrota, D.; Beňová, A.; Kalenská, D.; Kaplán, P. Role of Homocysteine in the Ischemic Stroke and Development of Ischemic Tolerance. Front. Neurosci. 2016, 10, 538. [Google Scholar]
- Tyagi, N.; Qipshidze, N.; Munjal, C.; Vacek, J.C.; Metreveli, N.; Givvimani, S.; Tyagi, S.C. Tetrahydrocurcumin ameliorates homocysteinylated cytochrome-c mediated autophagy in hyperhomocysteinemia mice after cerebral ischemia. J. Mol. Neurosci. 2012, 47, 128–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toda, N.; Okamura, T. Hyperhomocysteinemia impairs regional blood flow: Involvements of endothelial and neuronal nitric oxide. Pflugers Arch. 2016, 468, 1517–1525. [Google Scholar] [CrossRef]
- Kovalska, M.; Kovalska, L.; Mikuskova, K.; Adamkov, M.; Tatarkova, Z.; Lehotsky, J. p-ERKinvolvement in the neuroprotection exerted by ischemic preconditioning in rat hippocampus subjected to four vessel occlusion. J. Physiol. Pharmacol. 2014, 65, 767–776. [Google Scholar] [PubMed]
- Moreira, D.; Figueiró, P.W.; Siebert, C.; Prezzi, C.A.; Rohden, F.; Guma, F.C.; Manfredini, V.; Wyse, A.T. Chronic Mild Hyperhomocysteinemia Alters Inflammatory and Oxidative/Nitrative Status and Causes Protein/DNA Damage, as Well as Ultrastructural Changes in Cerebral Cortex: Is Acetylsalicylic Acid Neuroprotective? Neurotox. Res. 2018, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Kertowidjojo, E.; Ojaimi, C.; Martin-Fernandez, B.; Kandhi, S.; Wolin, M.; Hintze, T.H. Long-term methionine-diet induced mild hyperhomocysteinemia associated cardiac metabolic dysfunction in multiparous rats. Physiol. Rep. 2015, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, T.M.; Siebert, C.; de Oliveira, M.F.; Manfredini, V.; Wyse, A.T. Chronic mild Hyperhomocysteinemia impairs energy metabolism, promotes DNA damage and induces a Nrf2 response to oxidative stress in rats brain. Cell Mol. Neurobiol. 2019, 39, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Nuru, M.; Muradashvili, N.; Kalani, A.; Lominadze, D.; Tyagi, N. High methionine, low folate and low vitamin B6/B12 (HM-LF-LV) diet causes neurodegeneration and subsequent short-term memory loss. Metab. Brain Dis. 2018, 33, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Belloli, S.; Zanotti, L.; Murtaj, V.; Mazzon, C.; Di Grigoli, G.; Monterisi, C.; Masiello, V.; Iaccarino, L.; Cappelli, A.; Poliani, P.L.; et al. F-VC701-PET and MRI in the in vivo neuroinflammation assessment of a mouse model of multiple sclerosis. J. Neuroinflamm. 2018, 15, 33–44. [Google Scholar] [CrossRef]
- Johnson, K.A.; Fox, N.C.; Reisa, A.; Sperling, R.A.; Klunk, W.E. Brain Imaging in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012, 2, 1–23. [Google Scholar] [CrossRef]
- Hnilicová, P.; Kantorová, E.; Poláček, H.; Grendár, M.; Bittšanský, M.; Čierny, D.; Sivák, Š.; Zeleňák, K.; Lehotský, J.; Dobrota, D.; et al. Altered hypothalamic metabolism in early multiple sclerosis–MR spectroscopy study. J. Neurol. Sci. 2019, 407, 116458–116467. [Google Scholar] [CrossRef]
- Buffo, A.; Rolando, C.; Ceruti, S. Astrocytes in the damaged brain:molecular and cellular insights in to the irreactive response and healing potential. Biochem. Pharmacol. 2010, 79, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Lukaszevicz, A.C.; Sampaïo, N.; Guégan, C.; Benchoua, A.; Couriaud, C.; Chevalier, E.; Sola, B.; Lacombe, P.; Onténiente, B. High Sensitivity of Protoplasmic Cortical Astroglia to Focal Ischemia. J. Cerebral Blood Flow Metab. 2002, 22, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Gómez-Isla, T.; Growdon, J.H.; Frosch, M.P.; Hyman, B.T. A phenotypic change but not proliferation underlies glial responses in Alzheimer disease. Am. J. Pathol. 2013, 182, 2332–2344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dossi, E.; Vasile, F.; Rouach, N. Human astrocytes in the diseased brain. Brain Res. Bull. 2018, 136, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Weekman, E.M.; Woolums, A.E.; Sudduth, T.L.; Wilcock, D.M. Hyperhomocysteinemia-Induced Gene Expression Changes in the Cell Types of the Brain. ASN Neuro 2017, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Maler, J.M.; Seifert, W.; Hüther, G.; Wiltfang, J.; Rüther, E.; Kornhuber, J.; Bleich, S. Homocysteine induces cell death of ratastrocytes in vitro. Neurosci. Lett. 2003, 347, 85–88. [Google Scholar] [CrossRef]
- Cervetto, C.; Venturini, A.; Guidolin, D.; Maura, G.; Passalacqua, M.; Tacchetti, C.; Cortelli, P.; Genedani, S.; Candiani, S.; Ramoino, P.; et al. Homocysteine and A2A-D2 Receptor-Receptor Interaction at Striatal Astrocyte Processes. J. Mol. Neurosci. 2018, 65, 456–466. [Google Scholar] [CrossRef]
- Skowrońska, K.; Obara-Michlewska, M.; Zielińska, M.; Albrecht, J. NMDA Receptors in Astrocytes: In Search for Roles in Neurotransmission and Astrocytic Homeostasis. Int. J. Mol. Sci. 2019, 20, 309. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.C.; Tzeng, W.S.; Wang, C.C.; Cheng, B.C.; Chang, Y.K.; Chen, H.H.; Lin, P.C.; Huang, T.Y.; Chuang, T.J.; Lin, J.W.; et al. Neuroprotective effect of agmatine in rats with transient cerebral ischemia using MR imaging and histopathologic evaluation. Magn Reson Imaging 2013, 31, 1174–1181. [Google Scholar] [CrossRef]
- Öz, G.; Alger, J.R.; Barker, P.B.; Bartha, R.; Bizzi, A.; Boesch, C.; Bolan, P.J.; Brindle, K.M.; Cudalbu, C.; Dinçer, A.; et al. Clinical Proton MR spectroscopy in central nervous system Disorders. Radiology 2014, 270, 658–679. [Google Scholar]
- Soares, D.P.; Law, M. Magnetic resonance spectroscopy of the brain: Review of metabolites and clinical applications. Clin. Radiol. 2009, 64, 12–21. [Google Scholar] [CrossRef]
- Sager, T.N.; Laursen, H.; Fink-Jensen, A.; Topp, S.; Stensgaard, A.; Hedehus, M.; Rosenbaum, S.; Valsborg, J.S.; Hansen, A.J. N-Acetylaspartate Distribution in Rat Brain Striatum During Acute Brain Ischemia. J. Cerebral Blood Flow Metab. 1999, 19, 164–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, J.; Dogan, N.; Young, L.; Yang, F. Quantitative Radiomics: Impact of Pulse Sequence Parameter Selection on MRI-Based Textural Features of the Brain. Contrast Media Mol. Imaging 2018, 2018, 1729071–1729080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reischauer, C.; Gutzeit, A.; Neuwirth, C.; Fuchs, A.; Sartoretti-Schefer, S.; Weber, M.; Czell, D. In-vivo evaluation of neuronal and glial changes in amyotrophic lateral sclerosis with diffusion tensor spectroscopy. NeuroImage Clin. 2018, 20, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Le Stunff, H.; Véret, J.; Kassis, N.; Denom, J.; Meneyrol, K.; Paul, J.L.; Cruciani-Guglielmacci, C.; Magnan, C.; Janel, N. Deciphering the Link Between Hyperhomocysteinemia and Ceramide Metabolism in Alzheimer-Type Neurodegeneration. Front. Neurol. 2019, 10, 807–818. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Zhuo, J.; Racz, J.; Shi, D.; Roys, S.; Fiskum, G.; Gullapalli, R. Early microstructural and metabolic changes following controlled cortical impact injury in rat: A magnetic resonance imaging and spectroscopy study. J. Neurotrauma 2011, 28, 2091–2102. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.L.; Yeh, H.W.; Choi, I.Y.; Lee, P.; Berman, N.E.; Swerdlow, R.H.; Craciunas, S.C.; Brooks, W.M. Altered neurochemical profile after traumatic brain injury: H-MRS biomarkers of pathological mechanisms. J. Cereb Blood Flow Metab. 2012, 32, 2122–2134. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Ahram, A.; Berman, R.F.; Muizelaar, J.P.; Lyeth, B.G. Early loss of astrocytes after experimental Traumatic brain injury. Glia 2003, 44, 140–152. [Google Scholar] [CrossRef]
- Chang, L.; Munsaka, S.M.; Kraft-Terry, S.; Ernst, T. Magnetic resonance spectroscopy to assess neuroinflammation and neuropathic pain. J. Neuroimmune Pharmacol. 2013, 8, 576–593. [Google Scholar] [CrossRef]
- Harris, J.L.; In-Young Choi, I.Y.; Brooks, W.M. Probing astrocyte metabolism in vivo: Proton magnetic resonance spectroscopy in the injured and aging brain. Front. Aging Neurosci. 2015, 7, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Filibian, M.; Frasca, A.; Maggioni, D.; Micotti, E.; Vezzani, A.; Ravizza, T. In vivo imaging of glia activation using 1H-magnetic resonance spectroscopy to detect putative biomarkers of tissue epileptogenicity. Epilepsia 2012, 53, 1907–1916. [Google Scholar] [CrossRef]
- Chen, S.; Dong, Z.; Zhao, Y.; Sai, N.; Wang, X.; Liu, H.; Huang, G.; Zhang, Y. Homocysteine induces mitochondrial dysfunction involving the crosstalk between oxidative stress and mitochondrial pSTAT3 in rat ischemic brain. Sci. Rep. 2017, 7, 6932. [Google Scholar] [CrossRef] [PubMed]
- Kantarci, K.; Knopman, D.S.; Dickson, D.W.; Parisi, J.E.; Whitwell, J.L.; Weigand, S.D.; Josephs, K.A.; Boeve, B.F.; Petersen, R.C.; Jack, C.R., Jr. Alzheimer disease: Postmortem neuropathologic correlates of antemortem 1H MR spectroscopy metabolite measurements. Radiology 2008, 248, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Perła-Kaján, J.; Jakubowski, H. Dysregulation of Epigenetic Mechanisms of Gene Expression in the Pathologies of Hyperhomocysteinemia. Int. J. Mol. Sci. 2019, 20, 3140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merali, Z.; Huang, K.; Mikulis, D.; Silver, F.; Kassner, A. Evolution of blood-brain-barrier permeability after acute ischemic stroke. PLoS ONE 2017, 12, e0171558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.Y.; Wu, G.; Guo, S.X.; Geng, D.Y.; Li, J.L.; Yang, K. Multi-parameters of Magnetic Resonance Imaging to estimate Ischemia-Reperfusion Injury after stroke in Hyperglycemic Rats. Sci. Rep. 2019, 9, 2852–2870. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, L.; Miao, Y.; Yang, J.; Wang, X.; Wang, C.C.; Feng, J.; Wang, L. Homocysteine causes vascular endothelial dysfunction by disrupting endoplasmic reticulum redox homeostasis. Redox Biol. 2019, 20, 46–59. [Google Scholar] [CrossRef]
- Wang, X.; Cui, L.; Joseph, J.; Jiang, B.; Pimental, D.; Handy, D.E.; Liao, R.; Loscalzo, J. Homocysteine induces cardiomyocyte dysfunction and apoptosis through p38 MAPK-mediated increase in oxidant stress. J. Mol. Cell Cardiol. 2012, 52, 753–760. [Google Scholar] [CrossRef] [Green Version]
- Timkova, V.; Tatarkova, Z.; Lehotsky, J.; Racay, P.; Dobrota, D.; Kaplan, P. Effects of mild hyperhomocysteinemia on electron transport chain complexes, oxidative stress, and protein expression in rat cardiac mitochondria. Mol. Cell Biochem. 2016, 411, 261–270. [Google Scholar] [CrossRef]
- Majumder, A.; Singh, M.; George, A.K.; Homme, R.P.; Laha, A.; Tyagi, S.C. Remote ischemic conditioning as a cytoprotective strategy in vasculopathies during hyperhomocysteinemia: An emerging research perspective. J. Cell. Biochem. 2018, 120, 77–92. [Google Scholar] [CrossRef] [Green Version]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Soltanian-Zadeh, H.; Pasnoor, M.; Hammoud, R.; Jacobs, M.A.; Patel, S.C.; Mitsias, P.D.; Knight, R.A.; Zheng, Z.G.; Lu, M.; Chopp, M. MRI tissue characterization of experimental cerebral ischemia in rat. J. Magn. Reson. Imaging 2003, 17, 398–409. [Google Scholar] [CrossRef] [PubMed]






| Groups | C /n = 8/ | Met-C /n = 8/ | Met-IR-3d /n = 5/ | Met-IR-7d /n = 5/ | C/Met-C | C/Met-IR-3d | C/Met-IR-7d | Met-C/Met-IR-3d | Met-C/Met-IR-7d | Met-IR-3d/Met-IR-7d | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ratios | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||||||
| tNAA/tCr | 1.186 ± 0.079 | 1.189 ± 0.075 | 0.959 ± 0.106 | 1.034 ± 0.128 | 0.948 | 0.001 | 0.022 | 0.001 | 0.018 | 0.113 | |
| tCho/tCr | 0.203 ± 0.016 | 0.210 ± 0.022 | 0.201 ± 0.013 | 0.209 ± 0.021 | 0.456 | 0.880 | 0.522 | 0.426 | 0.930 | 0.439 | |
| mIns/tCr | 0.742 ± 0.058 | 0.746 ± 0.051 | 0.688 ± 0.082 | 0.828 ± 0.032 | 0.794 | 0.184 | 0.012 | 0.137 | 0.009 | 0.015 | |
| HIPPOCAMPUS | |||||
|---|---|---|---|---|---|
| Volume Change Mean ± SD | C (Volume Threshold) 91.7 mm3 | Met-C 10 ± 2% | Met-IR-3d 23 ± 6% | Met-IR-7d −6 ± 2% | |
| Tissue Volume Mean ± SD | |||||
| C 96.51 ± 4.78 mm3 | - | independent sample | |||
| Met-C 100.85 ± 1.82 mm3 | 0.031 | - | 2-tailed t-test | ||
| Met-IR-3d 112.81 ± 5.19 mm3 | <0.001 | <0.001 | - | ||
| Met-IR-7d 85.78 ± 2.01 mm3 | 0.001 | <0.001 | <0.001 | - | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovalska, M.; Hnilicova, P.; Kalenska, D.; Tomascova, A.; Adamkov, M.; Lehotsky, J. Effect of Methionine Diet on Time-Related Metabolic and Histopathological Changes of Rat Hippocampus in the Model of Global Brain Ischemia. Biomolecules 2020, 10, 1128. https://doi.org/10.3390/biom10081128
Kovalska M, Hnilicova P, Kalenska D, Tomascova A, Adamkov M, Lehotsky J. Effect of Methionine Diet on Time-Related Metabolic and Histopathological Changes of Rat Hippocampus in the Model of Global Brain Ischemia. Biomolecules. 2020; 10(8):1128. https://doi.org/10.3390/biom10081128
Chicago/Turabian StyleKovalska, Maria, Petra Hnilicova, Dagmar Kalenska, Anna Tomascova, Marian Adamkov, and Jan Lehotsky. 2020. "Effect of Methionine Diet on Time-Related Metabolic and Histopathological Changes of Rat Hippocampus in the Model of Global Brain Ischemia" Biomolecules 10, no. 8: 1128. https://doi.org/10.3390/biom10081128
APA StyleKovalska, M., Hnilicova, P., Kalenska, D., Tomascova, A., Adamkov, M., & Lehotsky, J. (2020). Effect of Methionine Diet on Time-Related Metabolic and Histopathological Changes of Rat Hippocampus in the Model of Global Brain Ischemia. Biomolecules, 10(8), 1128. https://doi.org/10.3390/biom10081128