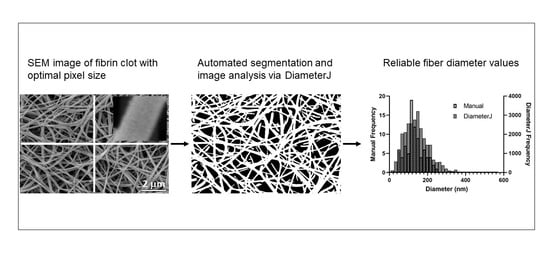
Automated Fiber Diameter and Porosity Measurements of Plasma Clots in Scanning Electron Microscopy Images
Abstract
:1. Introduction
2. Materials and Methods
2.1. Set 1: Postpartum Hemorrhage (PPH) Samples
2.1.1. Patient Population and Study Design
2.1.2. Plasma Preparation and Clinical Coagulation Testing
2.1.3. Scanning Electron Microscopy
2.2. Set 2: Acute Myocardial Infarction (AMI) Samples
2.2.1. Patient Population and Study Design
2.2.2. Plasma Preparation
2.2.3. Scanning Electron Microscopy
2.3. Set 3: Healthy Samples
2.3.1. Population and Study Design
2.3.2. Turbidity
2.3.3. Rheometry
2.3.4. Permeability
2.3.5. Scanning Electron Microscopy
2.4. Set 4: Systemic Lupus Erythematosus (SLE) Samples
2.4.1. Patient Population and Study Design
2.4.2. Scanning Electron Microscopy
2.5. Fiber Diameter Measurement
2.5.1. Manual Measurement of Fiber Diameter
2.5.2. DiameterJ-Based Fiber Diameter Measurements
2.6. DiameterJ-Based Network Porosity Measurements
3. Results
3.1. Comparison of Manual and Automated Fiber Diameter Measurements Using Three Large Data Sets
3.1.1. Set 1 Diameter Measurements
3.1.2. Set 2 Diameter Measurements
3.1.3. Set 3 Diameter Measurements
3.2. Detecting Differences between Patient and Healthy Control Plasma Clot Samples
3.2.1. Postpartum Hemorrhage, Sample Set 1
3.2.2. Systemic Lupus Erythematosus (SLE), Sample Set 4
3.3. Relation between Fibrin Fiber Diameter and Biophysical Clot Properties
3.4. Relation between Fibrin Network Porosity and Biophysical Clot Properties
4. Discussion
4.1. Optimal Image Parameters and Algorithms
4.2. Possible Reasons for Pixel Dependence of Diameter Measurement
4.3. Detecting Statistically Significant Differences in Patient Samples
4.4. Correlation of Diameter Measurements with Biophysical Clot Properties
4.5. Correlation between Fibrin Network Porosity and Biophysical Clot Properties
4.6. Limitation Due to Particles in Sample
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Badiei, N.; Sowedan, A.; Curtis, D.; Brown, M.; Lawrence, M.; Campbell, A.; Sabra, A.; Evans, P.; Weisel, J.; Chernysh, I.; et al. Effects of unidirectional flow shear stresses on the formation, fractal microstructure and rigidity of incipient whole blood clots and fibrin gels. Clin. Hemorheol. Microcirc. 2015, 60, 451–464. [Google Scholar] [CrossRef] [Green Version]
- Baheti, S.; Tunak, M. Characterization of fiber diameter using image analysis. IOP Conf. Ser. Mater. Sci. Eng. 2017, 254, 142002. [Google Scholar] [CrossRef]
- Kopytek, M.; Zabczyk, M.; Natorska, J.; Siudut, J.; Malinowski, K.P.; Ptaszek, P.; Glajcar, A.; Goralczyk, T.; Undas, A. Viscoelastic properties of plasma fibrin clots are similar in patients on rivaroxaban and vitamin K antagonists. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2019. [Google Scholar] [CrossRef]
- Langer, B.G.; Weisel, J.W.; Dinauer, P.; Nagaswami, C.; Bell, W.R. Deglycosylation of fibrinogen accelerates polymerization and increases lateral aggregation of fibrin fibers. J. Biol. Chem. 1988, 263, 15056–15063. [Google Scholar] [CrossRef]
- Li, W.; Sigley, J.; Pieters, M.; Helms, C.C.; Nagaswami, C.; Weisel, J.W.; Guthold, M. Fibrin Fiber Stiffness Is Strongly Affected by Fiber Diameter, but Not by Fibrinogen Glycation. Biophys. J. 2016, 110, 1400–1410. [Google Scholar] [CrossRef] [Green Version]
- Longstaff, C.; Thelwell, C.; Williams, S.C.; Silva, M.M.C.G.; Szabó, L.; Kolev, K. The interplay between tissue plasminogen activator domains and fibrin structures in the regulation of fibrinolysis: Kinetic and microscopic studies. Blood 2011, 117, 661–668. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Arotzena, O.; Meier, J.G.; Del Amo, C.; García-Aznar, J.M. Characterization of Fibrin and Collagen Gels for Engineering Wound Healing Models. Materials 2015, 8, 1636–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, D.M. Analysis of Fiber Network Architecture in Blood Vessels and Blood Clots. Master’s Thesis, San Jose State University, San Jose, CA, USA, 2019. [Google Scholar]
- Weisel, J.W.; Litvinov, R. Mechanisms of fibrin polymerization and clinical implications. Blood 2013, 121, 1712–1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collet, J.; Woodhead, J.; Soria, J.; Soria, C.; Mirshahi, M.; Caen, J.; Weisel, J. Fibrinogen Dusart: Electron microscopy of molecules, fibers and clots, and viscoelastic properties of clots. Biophys. J. 1996, 70, 500–510. [Google Scholar] [CrossRef] [Green Version]
- Collet, J.; Allali, Y.; Lesty, C.; Tanguy, M.; Silvain, J.; Ankri, A.; Blanchet, B.; Dumaine, R.; Gianetti, J.; Payot, L.; et al. Altered Fibrin Architecture Is Associated with Hypofibrinolysis and Premature Coronary Atherothrombosis. Arter. Thromb. Vasc. Biol. 2006, 26, 2567–2573. [Google Scholar] [CrossRef] [Green Version]
- Dunn, E.J.; Ariëns, R.A.S. Fibrinogen and Fibrin Clot Structure in Diabetes. Herz 2004, 29, 470–479. [Google Scholar] [CrossRef]
- Campbell, R.A.; Overmyer, K.A.; Selzman, C.H.; Sheridan, B.C.; Wolberg, A.S. Contributions of extravascular and intravascular cells to fibrin network formation, structure, and stability. Blood 2009, 114, 4886–4896. [Google Scholar] [CrossRef] [Green Version]
- Fatah, K.; Silveira, A.; Tornvall, P.; Karpe, F.; Blombäck, M.; Hamsten, A. Proneness to Formation of Tight and Rigid Fibrin Gel Structures in Men with Myocardial Infarction at a Young Age. Thromb. Haemost. 1996, 76, 535–540. [Google Scholar] [CrossRef]
- Machlus, K.; Cardenas, J.C.; Church, F.C.; Wolberg, A.S. Causal relationship between hyperfibrinogenemia, thrombosis, and resistance to thrombolysis in mice. Blood 2011, 117, 4953–4963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikovic, D.; Elezovic, I.; Zabczyk, M.; Hutenby, K.; Antovic, J.P.; Antovic, A. Improvement of fibrin clot structure after factor VIII injection in haemophilia A patients treated on demand. Thromb. Haemost. 2014, 111, 656–661. [Google Scholar] [CrossRef]
- Bridge, K.; Philippou, H.; Ariëns, R.A.S. Clot properties and cardiovascular disease. Thromb. Haemost. 2014, 112, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Dargaud, Y.; Prevost, C.; Lienhart, A.; Bordet, J.C.; Negrier, C. Evaluation of the overall haemostatic effect of recombinant factor VIIa by measuring thrombin generation and stability of fibrin clots. Haemophilia 2011, 17, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Hethershaw, E.; La Corte, A.L.C.; Duval, C.; Ali, M.; Grant, P.J.; Ariens, R.; Philippou, H. The effect of blood coagulation factor XIII on fibrin clot structure and fibrinolysis. J. Thromb. Haemost. JTH 2013, 12, 197–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leong, L.; Chernysh, I.N.; Xu, Y.; Sim, D.; Nagaswami, C.; De Lange, Z.; Kosolapova, S.; Cuker, A.; Kauser, K.; Weisel, J.W. Clot stability as a determinant of effective factor VIII replacement in hemophilia A. Res. Pract. Thromb. Haemost. 2017, 1, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Undas, A.; Ariëns, R.A. Fibrin Clot Structure and Function: A Role in the Pathophysiology of Arterial and Venous Thromboembolic Diseases, Arterioscler. Arter. Thromb. Vasc. Biol. 2011, 31, e88–e99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolberg, A.S.; Allen, G.A.; Monroe, D.M.; Hedner, U.; Roberts, H.R.; Hoffman, M. High dose factor VIIa improves clot structure and stability in a model of haemophilia B. Br. J. Haematol. 2005, 131, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Zucker, M.; Seligsohn, U.; Salomon, O.; Wolberg, A.S. Abnormal plasma clot structure and stability distinguish bleeding risk in patients with severe factor XI deficiency. J. Thromb. Haemost. JTH 2014, 12, 1121–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litvinov, R.I.; Nabiullina, R.M.; Zubairova, L.D.; Shakurova, M.A.; Andrianova, I.A.; Weisel, J.W. Lytic Susceptibility, Structure, and Mechanical Properties of Fibrin in Systemic Lupus Erythematosus. Front. Immunol. 2019, 10, 1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pretorius, E.; Steyn, H.; Engelbrecht, M.; Swanepoel, A.C.; Oberholzer, H.M. Differences in fibrin fiber diameters in healthy individuals and thromboembolic ischemic stroke patients. Blood Coagul. Fibrinolysis 2011, 22, 696–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siebenlist, K.R.; Mosesson, M.W.; Hernandez, I.; Bush, L.A.; Di Cera, E.; Shainoff, J.R.; Di Orio, J.P.; Stojanovic, L. Studies on the basis for the properties of fibrin produced from fibrinogen-containing γ′ chains. Blood 2005, 106, 2730–2736. [Google Scholar] [CrossRef]
- Hotaling, N.A.; Bharti, K.; Kriel, H.; Simon, C. DiameterJ: A validated open source nanofiber diameter measurement tool. Biomaterials 2015, 61, 327–338. [Google Scholar] [CrossRef] [Green Version]
- Hotaling, N.; Bharti, K.; Kriel, H.; Simon, C. Dataset for the validation and use of DiameterJ an open source nanofiber diameter measurement tool. Data Brief 2015, 5, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.G.; Song, J. Adopting automated image analysis tool for fibrin network: Can we obtain clot properties for practical application? Int. J. Lab. Hematol. 2017, 39, e121–e123. [Google Scholar] [CrossRef]
- Weisel, J.; Nagaswami, C. Computer modeling of fibrin polymerization kinetics correlated with electron microscope and turbidity observations: Clot structure and assembly are kinetically controlled. Biophys. J. 1992, 63, 111–128. [Google Scholar] [CrossRef] [Green Version]
- Siniarski, A.; Baker, S.R.; Duval, C.; Malinowski, K.P.; Gajos, G.; Nessler, J.; Ariëns, R.A. Quantitative analysis of clot density, fibrin fiber radius, and protofibril packing in acute phase myocardial infarction. Thromb. Res. 2021, 205, 110–119. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D. Third Universal Definition of Myocardial Infarction. Circulation 2012, 126, 2020–2035. [Google Scholar] [CrossRef] [Green Version]
- Teo, K.; Chow, C.K.; Vaz, M.; Rangarajan, S.; Yusufali, A. The Prospective Urban Rural Epidemiology (PURE) study: Examining the impact of societal influences on chronic noncommunicable diseases in low-, middle-, and high-income countries. Am. Hear. J. 2009, 158, 1–7.e1. [Google Scholar] [CrossRef] [PubMed]
- Pieters, M.; Philippou, H.; Undas, A.; De Lange, Z.; Rijken, D.; Mutch, N. An international study on the feasibility of a standardized combined plasma clot turbidity and lysis assay: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1007–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisman, T.; De Groot, P.G.; Meijers, J.C.M.; Rosendaal, F.R. Reduced plasma fibrinolytic potential is a risk factor for venous thrombosis. Blood 2005, 105, 1102–1105. [Google Scholar] [CrossRef] [Green Version]
- Pieters, M.; Undas, A.; Marchi, R.; DE Maat, M.P.M.; Weisel, J.W.; Ariëns, R.A.S. On behalf of the factor xiii and fibrinogen subcommittee of the scientific and standardisation committee of the international society for thrombosis and Haemostasis, an international study on the standardization of fibrin clot permeability measurement: Methodological considerations and implications for healthy control values. J. Thromb. Haemost. JTH 2012, 10, 2179–2181. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Chem. Biol. 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Ajjan, R.; Standeven, K.; Khanbhai, M.; Phoenix, F.; Gersh, K.; Weisel, J.; Kearney, M.; Ariëns, R.; Grant, P. Effects of Aspirin on Clot Structure and Fibrinolysis Using a Novel In Vitro Cellular System. Arter. Thromb. Vasc. Biol. 2009, 29, 712–717. [Google Scholar] [CrossRef] [Green Version]
- Collet, J.-P.; Moen, J.L.; Veklich, Y.I.; Gorkun, O.V.; Lord, S.T.; Montalescot, G.; Weisel, J.W. The αC domains of fibrinogen affect the structure of the fibrin clot, its physical properties, and its susceptibility to fibrinolysis. Blood 2005, 106, 3824–3830. [Google Scholar] [CrossRef] [PubMed]
- Hugenholtz, G.C.; Macrae, F.; Adelmeijer, J.; Dulfer, S.; Porte, R.J.; Lisman, T.; Ariens, R. Procoagulant changes in fibrin clot structure in patients with cirrhosis are associated with oxidative modifications of fibrinogen. J. Thromb. Haemost. JTH 2016, 14, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- Mavrides, E.; Allard, S.; Chandraharan, E.; Collins, P.; Green, L.; Hunt, B.J.; Riris, S.; Thomson, A.J. Prevention and Management of Postpartum Haemorrhage: Green-top Guideline No. 52. BJOG Int. J. Obstet. Gynaecol. 2016, 124, e106–e149. [Google Scholar] [CrossRef]
- Collet, J.P.; Park, D.; Lesty, C.; Soria, J.; Soria, C.; Montalescot, G.; Weisel, J.W. Influence of Fibrin Network Conformation and Fibrin Fiber Diameter on Fibrinolysis Speed. Arter. Thromb. Vasc. Biol. 2000, 20, 1354–1361. [Google Scholar] [CrossRef] [Green Version]
- Hudson, N.E. Biophysical Mechanisms Mediating Fibrin Fiber Lysis. BioMed Res. Int. 2017, 2017, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weigandt, K.M.; Pozzo, D.C.; Porcar, L. Structure of high density fibrin networks probed with neutron scattering and rheology. Soft Matter 2009, 5, 4321–4330. [Google Scholar] [CrossRef]
- Weisel, J.W.; Litvinov, R.I. Fibrin Formation, Structure and Properties. Subcell. Biochem. 2017, 82, 405–456. [Google Scholar] [CrossRef] [Green Version]
- Baker, S.R.; Zabczyk, M.; Macrae, F.L.; Duval, C.; Undas, A.; Ariëns, R.A.S. Recurrent venous thromboembolism patients form clots with lower elastic modulus than those formed by patients with non-recurrent disease. J. Thromb. Haemost. 2019, 17, 618–626. [Google Scholar] [CrossRef]
- Litvinov, R.I.; Weisel, J.W. What Is the Biological and Clinical Relevance of Fibrin? Semin. Thromb. Hemost. 2016, 42, 333–343. [Google Scholar] [CrossRef] [Green Version]
- Litvinov, R.I.; Weisel, J.W. Fibrin mechanical properties and their structural origins. Matrix Biol. 2016, 60-61, 110–123. [Google Scholar] [CrossRef]
- Wolberg, A.S.; Campbell, R.A. Thrombin generation, fibrin clot formation and hemostasis. Transfus. Apher. Sci. 2008, 38, 15–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhmurov, A.; Brown, A.E.; Litvinov, P.R.I.; Dima, R.I.; Weisel, P.J.W.; Barsegov, P.V. Molecular Structural Origins of Clot and Thrombus Mechanical Properties. Blood 2011, 118, 2257. [Google Scholar] [CrossRef]
- Lee, S.-J.J.; Nguyen, D.M.; Grewal, H.S.; Puligundla, C.; Saha, A.K.; Nair, P.M.; Cap, A.P.; Ramasubramanian, A.K. Image-based analysis and simulation of the effect of platelet storage temperature on clot mechanics under uniaxial strain. Biomech. Model. Mechanobiol. 2019, 19, 173–187. [Google Scholar] [CrossRef]
- Carr, M.E.; Hermans, J. Size and Density of Fibrin Fibers from Turbidity. Macromolecules 1978, 11, 46–50. [Google Scholar] [CrossRef]
- Dassi, C.; Seyve, L.; García, X.; Bigo, E.; Marlu, R.; Caton, F.; Polack, B. Fibrinography: A Multiwavelength Light-Scattering Assay of Fibrin Structure. HemaSphere 2019, 3, e166. [Google Scholar] [CrossRef] [PubMed]
- Ferri, F.; Calegari, G.R.; Molteni, M.; Cardinali, B.; Magatti, D.; Rocco, M. Size and Density of Fibers in Fibrin and Other Filamentous Networks from Turbidimetry: Beyond a Revisited Carr–Hermans Method, Accounting for Fractality and Porosity. Macromolecules 2015, 48, 5423–5432. [Google Scholar] [CrossRef]
- Pieters, M.; Guthold, M.; Nunes, C.M.; De Lange, Z. Interpretation and Validation of Maximum Absorbance Data Obtained from Turbidimetry Analysis of Plasma Clots. Thromb. Haemost. 2019, 120, 044–054. [Google Scholar] [CrossRef] [PubMed]
- Yeromonahos, C.; Polack, B.; Caton, F. Nanostructure of the Fibrin Clot. Biophys. J. 2010, 99, 2018–2027. [Google Scholar] [CrossRef] [Green Version]
- Undas, A. Prothrombotic Fibrin Clot Phenotype in Patients with Deep Vein Thrombosis and Pulmonary Embolism: A New Risk Factor for Recurrence. BioMed Res. Int. 2017, 2017, 1–9. [Google Scholar] [CrossRef]



| Characteristics | Women with Severe PPH (n = 10) | Women without PPH (n = 5) | p |
|---|---|---|---|
| Age, year | 31 (25–33) | 43 (36–44) | 0.01 |
| N of pregnancies | 3 (2–4) | 2 (1–3) | 0.61 |
| N of miscarriages | 1 (0–2) | 0 (0–1) | 0.24 |
| Blood loss, mL | 3000 (2000–7000) | <500 | n/a |
| Blood type O, % | 33% | 50% | 0.57 |
| Abnormal bleeding score, % | 60% | 0% | 0.03 |
| Algorithms | S4 | M8 | T4 | S8 | T8 | T7 | M7 | T2 |
|---|---|---|---|---|---|---|---|---|
| Average deviations from arithmetic mean (%) | −13.03 | −12.33 | −12.29 | −8.46 | −6.16 | −6.09 | −5.91 | −5.72 |
| Algorithms | M4 | T6 | T3 | M6 | M5 | T1 | T5 | S3 |
| Average deviations from arithmetic mean (%) | −4.24 | −2.72 | −1.87 | −1.76 | −1.37 | −1.11 | 4.77 | 5.09 |
| Algorithms | S1 | S7 | M3 | S2 | S5 | S6 | M1 | M2 |
| Average deviations from arithmetic mean (%) | 7.37 | 7.61 | 8.47 | 8.76 | 8.93 | 11.06 | 11.63 | 13.80 |
| Type of Measurement | Manual | M6 | M7 | M3 | M5 | T2 | T6 | T3 | T4 | T5 | S7 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arithmetic Mean for set 1; N = 120 SEM images (nm) | 133 | 132 | 127 | 140 | 133 | 128 | 130 | 132 | 120 | 138 | 142 |
| Correlation r * with Manual Method | - | 0.87 | 0.86 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 |
| Manual | S2 | M2 | M1 | S5 | S6 | S1 | M3 | M4 | S7 | S3 | |
| Arithmetic Mean for set 2; N = 69 SEM images (nm) | 127 | 122 | 131 | 128 | 123 | 127 | 121 | 128 | 111 | 124 | 119 |
| Correlation r * with Manual Method | 0.77 | 0.76 | 0.76 | 0.75 | 0.75 | 0.73 | 0.72 | 0.71 | 0.71 | 0.66 | |
| Manual | T6 | T1 | M5 | T2 | M7 | S3 | M3 | T7 | S7 | T8 | |
| Arithmetic Mean for set 3; N = 150 SEM images (nm) | 191 | 249 | 253 | 253 | 235 | 235 | 250 | 254 | 245 | 253 | 244 |
| Correlation r * with Manual Method | - | 0.77 | 0.74 | 0.74 | 0.73 | 0.73 | 0.72 | 0.71 | 0.70 | 0.70 | 0.68 |
| Type of Measurement | Manual | T1 | S5 | M6 | S3 | M1 | S7 | M3 | T5 | S1 | T6 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Histogram Mean for set 1; N = 120 SEM images (nm) | 133 | 122 | 135 | 121 | 129 | 135 | 131 | 130 | 126 | 135 | 120 |
| Correlation r * with Manual Method | - | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.84 | 0.84 | 0.82 | 0.82 | 0.81 |
| Manual | S1 | M2 | S5 | M1 | S2 | S6 | M3 | S7 | S3 | M4 | |
| Histogram Mean for set 2; N = 69 SEM images (nm) | 127 | 108 | 116 | 110 | 115 | 109 | 113 | 113 | 111 | 106 | 100 |
| Correlation r * with Manual Method | 0.69 | 0.69 | 0.68 | 0.67 | 0.67 | 0.66 | 0.66 | 0.62 | 0.61 | 0.60 | |
| Manual | T6 | S3 | M5 | M3 | T1 | T2 | T7 | S7 | T8 | M7 | |
| Histogram Mean for set 3; N = 150 SEM images (nm) | 191 | 225 | 222 | 229 | 227 | 228 | 214 | 223 | 221 | 221 | 214 |
| Correlation r * with Manual Method | - | 0.82 | 0.80 | 0.79 | 0.79 | 0.79 | 0.79 | 0.78 | 0.79 | 0.74 | 0.73 |
| Type of Measurement | Manual | T6 | T5 | S3 |
|---|---|---|---|---|
| Arithmetic Mean; N = 11 | 178 | 191 | 234 | 230 |
| Correlation r(p) between the arithmetic mean and manual measurement | - | 0.73 (0.01) | 0.68 (0.02) | 0.83 (0.001) |
| Histogram Mean; N = 11 | 178 | 192 | 206 | 197 |
| Correlation r(p) between the histogram mean and manual measurement | - | 0.80 (0.003) | 0.72 (0.01) | 0.78 (0.004) |
| Variable | Fibrinogen Concentration r (p) | Maximum Absorbance r (p) | Permeability (Ks) r (p) | Storage Modulus (G’) r (p) | Loss Modulus (G”) r (p) | Tan Delta (G’/G”) r (p) |
|---|---|---|---|---|---|---|
| Diameter | ||||||
| Manual | 0.44 (0.01) | 0.46 (0.01) | −0.04 (0.9) | 0.27 (0.2) | 0.29 (0.1) | −0.06 (0.7) |
| M8 | 0.51 (0.01) | 0.57 (0.006) | −0.16 (0.47) | 0.30 (0.18) | 0.29 (0.19) | −0.13 (0.57) |
| T4 | 0.58 (0.003) | 0.58 (0.003) | −0.12 (0.58) | 0.23 (0.27) | 0.21 (0.32) | −0.12 (0.59) |
| T6 | 0.33 (0.07) | 0.43 (0.02) | 0.03 (0.87) | 0.28 (0.14) | 0.24 (0.20) | −0.06 (0.73) |
| T5 | 0.32 (0.08) | 0.42 (0.02) | 0.071 (0.70) | 0.34 (0.07) | 0.28 (0.13) | −0.20 (0.28) |
| S6 | 0.25 (0.18) | 0.37 (0.05) | 0.15 (0.43) | 0.34 (0.07) | 0.30 (0.10) | −0.18 (0.35) |
| M6 | 0.26 (0.17) | 0.35 (0.06) | 0.12 (0.52) | 0.27 (0.15) | 0.20 (0.27) | −0.16 (0.40) |
| Porosity | ||||||
| Permeability Ks (cm2 × 10−9) | −0.54 (0.003) | −0.67 (<0.0001) | - | −0.48 (0.007) | −0.51 (0.004) | −0.18 (0.4) |
| T6 Mean pore area (µm2) | −0.14 (0.43) | 0.08 (0.67) | 0.37(0.03) | 0.22 (0.24) | 0.19 (0.32) | −0.15 (0.17) |
| T5 Mean pore area (µm2) | −0.13 (0.49) | 0.08 (0.67) | 0.41 (0.02) | 0.23 (0.23) | 0.22 (0.24) | −0.19 (0.32) |
| S6 Mean pore area (µm2) | −0.11 (0.55) | 0.10 (0.61) | 0.25 (0.18) | 0.21 (0.25) | 0.21 (0.27) | −0.00 (0.10) |
| M6 Mean pore area (µm2) | −0.03 (0.88) | 0.24 (0.20) | 0.10 (0.61) | 0.21 (0.28) | 0.23 (0.23) | 0.06 (0.77) |
| T6 Porosity (%) | −0.42 (0.02) | −0.25 (0.18) | 0.41 (0.02) | 0.07 (0.71) | 0.08 (0.67) | −0.20 (0.27) |
| T5 Porosity (%) | −0.50 (0.004) | −0.23 (0.20) | 0.42 (0.20) | −0.05 (0.78) | −0.01 (0.96) | −0.05 (0.79) |
| S6 Porosity (%) | −0.26 (0.17) | −0.28 (0.13) | 0.06 (0.75) | −0.17 (0.36) | −0.16 (0.41) | 0.12 (0.51) |
| M6 Porosity (%) | −0.05 (0.80) | 0.1 (0.62) | −0.03 (0.89) | 0.08 (0.68) | 0.13 (0.49) | 0.09 (0.64) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daraei, A.; Pieters, M.; Baker, S.R.; de Lange-Loots, Z.; Siniarski, A.; Litvinov, R.I.; Veen, C.S.B.; de Maat, M.P.M.; Weisel, J.W.; Ariëns, R.A.S.; et al. Automated Fiber Diameter and Porosity Measurements of Plasma Clots in Scanning Electron Microscopy Images. Biomolecules 2021, 11, 1536. https://doi.org/10.3390/biom11101536
Daraei A, Pieters M, Baker SR, de Lange-Loots Z, Siniarski A, Litvinov RI, Veen CSB, de Maat MPM, Weisel JW, Ariëns RAS, et al. Automated Fiber Diameter and Porosity Measurements of Plasma Clots in Scanning Electron Microscopy Images. Biomolecules. 2021; 11(10):1536. https://doi.org/10.3390/biom11101536
Chicago/Turabian StyleDaraei, Ali, Marlien Pieters, Stephen R. Baker, Zelda de Lange-Loots, Aleksander Siniarski, Rustem I. Litvinov, Caroline S. B. Veen, Moniek P. M. de Maat, John W. Weisel, Robert A. S. Ariëns, and et al. 2021. "Automated Fiber Diameter and Porosity Measurements of Plasma Clots in Scanning Electron Microscopy Images" Biomolecules 11, no. 10: 1536. https://doi.org/10.3390/biom11101536
APA StyleDaraei, A., Pieters, M., Baker, S. R., de Lange-Loots, Z., Siniarski, A., Litvinov, R. I., Veen, C. S. B., de Maat, M. P. M., Weisel, J. W., Ariëns, R. A. S., & Guthold, M. (2021). Automated Fiber Diameter and Porosity Measurements of Plasma Clots in Scanning Electron Microscopy Images. Biomolecules, 11(10), 1536. https://doi.org/10.3390/biom11101536