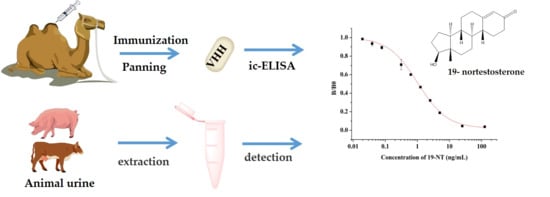
Nanobody-Based Indirect Competitive ELISA for Sensitive Detection of 19-Nortestosterone in Animal Urine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Construction of Phage Displayed Nanobody Library of Nanobody Library.
2.3. Construction and Screening of Phage Display Library
2.4. Preparation and Identification of Anti-19-NT Nanobody
2.5. Stability Analysis of Anti-19-NT Nanobody
2.6. Development and Optimization of ic-ELISA Based on Nanobody
2.7. Real Sample Analysis by ic-ELISA and UPLC-MS/MS
3. Results and Discussion
3.1. Screening and Identification of Nanobody
3.2. Stability Analysis of Anti-19-NT Nanobody
3.3. Establishment of ic-ELISA Method for 19-NT Based on Nanobody
3.4. Optimization of ic-ELISA
3.5. Sample Analysis by ic-ELISA and UPLC-MS/MS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Kang, Z.-S.; Li, H. Determination of Nine Steroid Hormone Residues in Beef Samples by Gel Permeation Chromatography-Solid Phase Extraction-Rapid Resolution Liquid Chromatography-Mass Spectrometry/Mass Spectrometry. Chin. J. Anal. Chem. 2010, 38, 1272–1276. [Google Scholar] [CrossRef]
- Bhasin, S.; Jasuja, R. Selective androgen receptor modulators as function promoting therapies. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 232–240. [Google Scholar] [CrossRef] [Green Version]
- Alireza Shirpoor, B.H.A.T.; Fatemeh Kheradmand, M.Z. Nandrolone administration with or without strenuous exercise increases cardiac fatal genes overexpression, calcium/calmodulin-dependent protein kinaseiiδ, and monoamine oxidase activities and enhances blood pressure in adult wistar rats. Gene 2019, 697, 131–137. [Google Scholar] [CrossRef]
- Seara, F.A.; Olivares, E.L.; Nascimento, J.H. Anabolic steroid excess and myocardial infarction: From ischemia to reperfusion injury. Steroids 2020, 161, 108660. [Google Scholar] [CrossRef]
- Divari, S.; Berio, E.; Pregel, P.; Sereno, A.; Chiesa, L.; Pavlovic, R.; Panseri, S.; Bovee, T.F.; Biolatti, B.; Cannizzo, F.T. Effects and detection of Nandrosol and ractopamine administration in veal calves. Food Chem. 2017, 221, 706–713. [Google Scholar] [CrossRef]
- Bovee, T.H.F.; Bor, G.; Heskamp, H.H.; Lasaroms, J.J.; Sanders, M.B.; Nielen, M.W.F. Validation and application of a yeast bioassay for screening androgenic activity in calf urine and feed. Anal. Chim. Acta 2009, 637, 225–234. [Google Scholar] [CrossRef]
- Ahmadkhaniha, R.; Kobarfard, F.; Rastkari, N.; Khoshayand, M.; Amini, M.; Shafiee, A. Assessment of endogenous androgen levels in meat, liver and testis of Iranian native cross-breed male sheep and bull by gas chromatography-mass spectrometry. Food Addit. Contam. Part A 2009, 26, 453–465. [Google Scholar] [CrossRef]
- González-Martínez, M.Á.; Puchades, R.; Maquieira, Á. Modern Techniques for Food Authentication, 2nd ed.; Sun, D., Ed.; Academic Press: Amsterdam, The Netherlands, 2018; pp. 617–657. [Google Scholar]
- Zhang, Z.; Zeng, K.; Liu, R. Immunochemical detection of emerging organic contaminants in environmental waters. TrAC Trends Anal. Chem. 2017, 87, 49–57. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Z.; Zhang, H.; Zhang, X.; Liu, X.; Wang, S. Monoclonal Antibody-Based ELISA and Colloidal Gold Immunoassay for Detecting 19-Nortestosterone Residue in Animal Tissues. J. Agric. Food Chem. 2011, 59, 9763–9769. [Google Scholar] [CrossRef]
- Roda, A.; Manetta, A.C.; Portanti, O.; Mirasoli, M.; Guardigli, M.; Pasini, P.; Lelli, R. A rapid and sensitive 384-well microtitre for matchemiluminescent enzyme immunoassay for 19-nortestosterone. Luminescence 2003, 18, 72–78. [Google Scholar] [CrossRef]
- Holubová, B.; Göselová, S.; Sevcikova, L.; Vlach, M.; Blažková, M.; Lapčík, O.; Fukal, L. Rapid immunoassays for detection of anabolic nortestosterone in dietary supplements. Czech J. Food Sci. 2013, 31, 514–519. [Google Scholar] [CrossRef] [Green Version]
- Muyldermans, S. Nanobodies: Natural Single-Domain Antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef] [Green Version]
- He, T.; Zhu, J.; Nie, Y.; Hu, R.; Wang, T.; Li, P.; Zhang, Q.; Yang, Y. Nanobody Technology for Mycotoxin Detection: Current Status and Prospects. Toxins 2018, 10, 180. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Li, Z.-F.; Yang, Y.-Y.; Wan, D.-B.; Vasylieva, N.; Zhang, Y.-Q.; Cai, J.; Wang, H.; Shen, Y.-D.; Xu, Z.-L.; et al. Chemiluminescent Enzyme Immunoassay and Bioluminescent Enzyme Immunoassay for Tenuazonic Acid Mycotoxin by Exploitation of Nanobody and Nanobody–Nanoluciferase Fusion. Anal. Chem. 2020, 92, 11935–11942. [Google Scholar] [CrossRef]
- Ren, W.; Li, Z.; Xu, Y.; Wan, D.; Barnych, B.; Li, Y.; Tu, Z.; He, Q.; Fu, J.; Hammock, B.D. One-Step Ultrasensitive Bioluminescent Enzyme Immunoassay Based on Nanobody/Nanoluciferase Fusion for Detection of Aflatoxin B1 in Cereal. J. Agric. Food Chem. 2019, 67, 5221–5229. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Xu, Z.-L.; Wang, F.; Cai, J.; Dong, J.-X.; Zhang, J.-R.; Si, R.; Wang, C.-L.; Wang, Y.; Shen, Y.-D.; et al. Isolation of Bactrian Camel Single Domain Antibody for Parathion and Development of One-Step dc-FEIA Method Using VHH-Alkaline Phosphatase Fusion Protein. Anal. Chem. 2018, 90, 12886–12892. [Google Scholar] [CrossRef]
- Zhang, J.-R.; Wang, Y.; Dong, J.-X.; Yang, J.; Zhang, Y.-Q.; Wang, F.; Si, R.; Xu, Z.; Xu, Z.; Xiao, Z.-L.; et al. Development of a Simple Pretreatment Immunoassay Based on an Organic Solvent-Tolerant Nanobody for the Detection of Carbofuran in Vegetable and Fruit Samples. Biomolecules 2019, 9, 576. [Google Scholar] [CrossRef] [Green Version]
- Pírez-Schirmer, M.; Rossotti, M.; Badagian, N.; Leizagoyen, C.; Brena, B.M.; González-Sapienza, G. Comparison of Three Antihapten VHH Selection Strategies for the Development of Highly Sensitive Immunoassays for Microcystins. Anal. Chem. 2017, 89, 6800–6806. [Google Scholar] [CrossRef]
- Zhang, Y.; He, F.; Wan, Y.; Meng, M.; Xu, J.; Yi, J.; Wang, Y.; Feng, C.; Wang, S.; Xi, R. Generation of anti-trenbolone monoclonal antibody and establishment of an indirect competitive enzyme-linked immunosorbent assay for detection of trenbolone in animal tissues, feed and urine. Talanta 2011, 83, 732–737. [Google Scholar] [CrossRef]
- Ebrahimizadeh, W.; Gargari, S.M.; Rajabibazl, M.; Ardekani, L.S.; Zare, H.; Bakherad, H. Isolation and characterization of protective anti-LPS nanobody against V. cholerae O1 recognizing Inaba and Ogawa serotypes. Appl. Microbiol. Biotechnol. 2012, 97, 4457–4466. [Google Scholar] [CrossRef]
- Olichon, A.; Schweizer, D.; Muyldermans, S.; De Marco, A. Heating as a rapid purification method for recovering correctly-folded thermotolerant VH and VHH domains. BMC Biotechnol. 2007, 7, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, D.; Li, G.; Hu, H.; Xue, H.; Zhang, M.; Zhu, M.; Gong, X.; Kennis, J.T.M.; Wan, Y.; Shen, Y. Direct Immunoassay for Facile and Sensitive Detection of Small Molecule Aflatoxin B1 based on Nanobody. Chem. A Eur. J. 2018, 24, 9869–9876. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, R.; Kitabatake, N. Heat-induced formation of intermolecular disulfide linkages between thaumatin molecules that do not contain cysteine residues. J. Agric. Food Chem. 1999, 47, 4950–4955. [Google Scholar] [CrossRef] [PubMed]
- Zabetakis, D.; Olson, M.A.; Anderson, G.P.; Legler, P.M.; Goldman, E.R. Evaluation of Disulfide Bond Position to Enhance the Thermal Stability of a Highly Stable Single Domain Antibody. PLoS ONE 2014, 9, e115405. [Google Scholar] [CrossRef] [PubMed]
- Akazawa-Ogawa, Y.; Uegaki, K.; Hagihara, Y. The role of intra-domain disulfide bonds in heat-induced irreversible denaturation of camelid single domain VHH antibodies. J. Biochem. 2015, 159, 111–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumoulin, M.; Conrath, K.; Van Meirhaeghe, A.; Meersman, F.; Heremans, K.; Frenken, L.G.; Muyldermans, S.; Wyns, L.; Matagne, A. Single-domain antibody fragments with high conformational stability. Protein Sci. 2009, 11, 500–515. [Google Scholar] [CrossRef]
- Akazawa-Ogawa, Y.; Takashima, M.; Lee, Y.-H.; Ikegami, T.; Goto, Y.; Uegaki, K.; Hagihara, Y. Heat-induced Irreversible Denaturation of the Camelid Single Domain VHH Antibody Is Governed by Chemical Modifications. J. Biol. Chem. 2014, 289, 15666–15679. [Google Scholar] [CrossRef] [Green Version]
- Goldman, E.R.; Liu, J.L.; Zabetakis, D.; Anderson, G.P. Enhancing Stability of Camelid and Shark Single Domain Antibodies: An Overview. Front. Immunol. 2017, 8, 865. [Google Scholar] [CrossRef] [Green Version]
- Horáček, J.; Skladal, P. Effect of organic solvents on immunoassays of environmental pollutants studied using a piezoelectric biosensor. Anal. Chim. Acta 2000, 412, 37–45. [Google Scholar] [CrossRef]
- Timson, D.J. Four Challenges for Better Biocatalysts. Fermentation 2019, 5, 39. [Google Scholar] [CrossRef] [Green Version]
- Muyldermans, S.; Cambillau, C.; Wyns, L. Recognition of antigens by single-domain antibody fragments: The superfluous luxury of paired domains. Trends Biochem. Sci. 2001, 26, 230–235. [Google Scholar] [CrossRef]
- Bever, C.S.; Dong, J.-X.; Vasylieva, N.; Barnych, B.; Cui, Y.; Xu, Z.-L.; Hammock, B.D.; Gee, S.J. VHH antibodies: Emerging reagents for the analysis of environmental chemicals. Anal. Bioanal. Chem. 2016, 408, 5985–6002. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Conneely, G.; Crowe, M.A.; Aherne, M.; Pravda, M.; Guilbault, G.G. Screening for testosterone, methyltestosterone, 19-nortestosterone residues and their metabolites in bovine urine with enzyme-linked immunosorbent assay (ELISA). Anal. Chim. Acta 2006, 570, 116–123. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, H.; Li, G.; Wang, Z.; Wang, J.; Zhao, H. Preparation of Anti-Nortestosterone Antibodies and Development of an Indirect Heterologous Competitive Enzyme-Linked Immunosorbent Assay to Detect Nortestosterone Residues in Animal Urine. Anal. Lett. 2011, 44, 2373–2393. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, L.Q.; Peng, C.; Chen, Z.; Xu, C. A new development of measurement of 19-Nortestosterone by combining immunochromatographic strip assay and ImageJ software. Food Agric. Immunol. 2009, 20, 1–10. [Google Scholar] [CrossRef]
- Zhao, F.; Tian, Y.; Shen, Q.; Liu, R.; Shi, R.; Wang, H.; Yang, Z. A novel nanobody and mimotope based immunoassay for rapid analysis of aflatoxin B1. Talanta 2019, 195, 55–61. [Google Scholar] [CrossRef]











| Analytes | Structure | Nb2F7 (CR) | Nb2B8 (CR) | Nb3D10 (CR) |
|---|---|---|---|---|
| 19-NT |  | 100% | 100% | 100% |
| TER |  | 5.67% | 45.32% | 8.49% |
| E2 |  | 0.19% | 38.48% | <0.1% |
| TRE |  | 11.24% | 22.47% | 13.52% |
| MT |  | <0.1% | <0.1% | <0.1% |
| TP |  | <0.1% | <0.1% | <0.1% |
| NPP |  | <0.1% | <0.1% | <0.1% |
| Antibody | Detection Method | IC50 (ng/mL) | LOD (ng/mL) | Reference |
|---|---|---|---|---|
| pAb | ic-ELISA | 27 | 1.9 | [28] |
| pAb | ELISA | 1.97 | 0.013 | [29] |
| pAb | ELISA | 6.41 | 0.09 | [30] |
| pAb | strip | - | 5 | [31] |
| mAb | ic-ELISA | 0.55 | 0.002 | [10] |
| Nb | ic-ELISA | 1.02 | 0.10 | this work |
| Sample | Spiked Level (ng/mL) | Found ± SD (ng/mL) | Recovery (%) | CV (%) |
|---|---|---|---|---|
| bovine urine | 5 | 4.64 ± 0.66 | 92.82 | 14.25 |
| 10 | 8.67 ± 0.68 | 86.87 | 7.82 | |
| 40 | 39.69 ± 1.39 | 99.24 | 3.50 | |
| pig urine | 10 | 8.26 ± 0.69 | 82.61 | 8.39 |
| 20 | 17.07 ± 0.50 | 86.87 | 6.43 | |
| 80 | 75.26 ± 1.16 | 94.08 | 2.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.-y.; Wang, Y.; Zhang, Y.-f.; Wang, F.; Liang, Y.-f.; Yang, J.-y.; Xu, Z.-l.; Shen, Y.-d.; Wang, H. Nanobody-Based Indirect Competitive ELISA for Sensitive Detection of 19-Nortestosterone in Animal Urine. Biomolecules 2021, 11, 167. https://doi.org/10.3390/biom11020167
Yang Y-y, Wang Y, Zhang Y-f, Wang F, Liang Y-f, Yang J-y, Xu Z-l, Shen Y-d, Wang H. Nanobody-Based Indirect Competitive ELISA for Sensitive Detection of 19-Nortestosterone in Animal Urine. Biomolecules. 2021; 11(2):167. https://doi.org/10.3390/biom11020167
Chicago/Turabian StyleYang, Yuan-yuan, Yu Wang, Yi-feng Zhang, Feng Wang, Yi-fan Liang, Jin-yi Yang, Zhen-lin Xu, Yu-dong Shen, and Hong Wang. 2021. "Nanobody-Based Indirect Competitive ELISA for Sensitive Detection of 19-Nortestosterone in Animal Urine" Biomolecules 11, no. 2: 167. https://doi.org/10.3390/biom11020167
APA StyleYang, Y. -y., Wang, Y., Zhang, Y. -f., Wang, F., Liang, Y. -f., Yang, J. -y., Xu, Z. -l., Shen, Y. -d., & Wang, H. (2021). Nanobody-Based Indirect Competitive ELISA for Sensitive Detection of 19-Nortestosterone in Animal Urine. Biomolecules, 11(2), 167. https://doi.org/10.3390/biom11020167