Unaltered Liver Regeneration in Post-Cholestatic Rats Treated with the FXR Agonist Obeticholic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
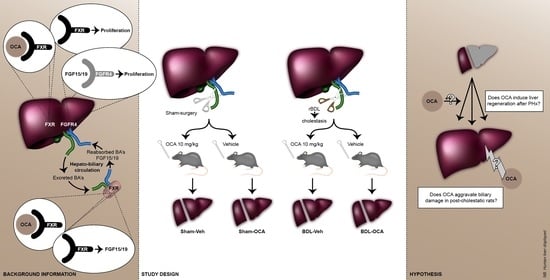
2.2. Experimental Design
2.3. Indocyanine Green Liver Function Test
2.4. Histology
2.5. Clinical Chemistry
2.6. Quantitative Real-Time Polymerase Chain Reaction
2.7. Mass Spectrometry
2.8. Patient Data
2.9. Statistical Analysis
3. Results
3.1. Obstructive Cholestasis Following Reversible Bile Duct Ligation
3.2. Liver Mass Prior to Partial Hepatectomy
3.3. Changes in mRNA Expression Prior to Partial Hepatectomy in Cholestatic Rats
3.4. Liver Regeneration after Partial Hepatectomy
3.5. Effects of OCA on Liver Regeneration after PHx
3.6. Changes in Gene Expression after PHx and as Result of OCA Treatment
3.7. OCA Treatment and Biliary Injury in Post-Cholestatic Rats
3.8. Bile Acid Pool Composition
4. Discussion
4.1. Obstructive Cholestasis Decreases Proliferative Signaling through Intestinal FXR but Does Not Increase Signaling through Hepatic FXR
4.2. The Increased Liver Weight after BDL Is Not the Result of Proliferation
4.3. Restoration of Intestinal Bile Flow 1 Day before PHx allows for Adequate Regenerative Signaling through FXR after PHx
4.4. OCA Does Not Affect Liver Regeneration after Obstructive Cholestasis but Accelerates Liver Regeneration in Non-Cholestatic Rats
4.5. OCA Did Not Aggravate Hepatobiliary Injury Because BSEP Was Not Upregulated
4.6. Influence of OCA on BA Homeostasis
4.7. OCA Does Not Affect ICG Clearance and Transporters in Post-Cholestatic and Non-Cholestatic Rats
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Haan, L.; van der Lely, S.J.; Warps, A.K.; Hofsink, Q.; Olthof, P.B.; de Keijzer, M.J.; Lionarons, D.A.; Mendes-Dias, L.; Bruinsma, B.G.; Uygun, K.; et al. Post-hepatectomy liver regeneration in the context of bile acid homeostasis and the gutliver signaling axis. J. Clin. Transl. Res. 2018, 4, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Michalopoulos, G.K. Hepatostat: Liver regeneration and normal liver tissue maintenance. Hepatology 2017, 65, 1384–1392. [Google Scholar] [CrossRef]
- Taub, R. Liver regeneration 4: Transcriptional control of liver regeneration. FASEB J. 1996, 10, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Taub, R. Liver regeneration: From myth to mechanism. Nat. Rev. Mol. Cell Biol. 2004, 5, 836–847. [Google Scholar] [CrossRef]
- Hoekstra, L.T.; de Graaf, W.; Nibourg, G.A.A.; Heger, M.; Bennink, R.J.; Stieger, B.; van Gulik, T.M. Physiological and Biochemical Basis of Clinical Liver Function Tests. Ann. Surg. 2013, 257, 27–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olthof, P.B.; Coelen, R.J.S.; Bennink, R.J.; Heger, M.; Lam, M.F.; Besselink, M.G.; Busch, O.R.; van Lienden, K.P.; van Gulik, T.M. 99mTc-mebrofenin hepatobiliary scintigraphy predicts liver failure following major liver resection for perihilar cholangiocarcinoma. HPB 2017, 19, 850–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kambakamba, P.; DeOliveira, M.L. Perihilar cholangiocarcinoma: Paradigms of surgical management. Am. J. Surg. 2014, 208, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Olthof, P.B.; Coelen, R.J.; Heger, M.; Klümpen, H.; Rauws, E.A.; van Gulik, T.M. Emerging local ablative therapies for unresectable perihilar cholangiocarinoma: Time for re-appraisal. United Eur. Gastroenterol. J. 2017, 5, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Olthof, P.B.; Coelen, R.J.; Wiggers, J.K.; Besselink, M.G.; Busch, O.R.; van Gulik, T.M. External biliary drainage following major liver resection for perihilar cholangiocarcinoma: Impact on development of liver failure and biliary leakage. HPB 2016, 18, 348–353. [Google Scholar] [CrossRef] [Green Version]
- Kloek, J.J.; Heger, M.; van der Gaag, N.A.; Beuers, U.; van Gulik, T.M.; Gouma, D.J.; Levi, M. Effect of Preoperative Biliary Drainage on Coagulation and Fibrinolysis in Severe Obstructive Cholestasis. J. Clin. Gastroenterol. 2010, 44, 646–652. [Google Scholar] [CrossRef] [Green Version]
- Kloek, J.J.; Maréchal, X.; Roelofsen, J.; Houtkooper, R.H.; van Kuilenburg, A.B.; Kulik, W.; Bezemer, R.; Neviere, R.; van Gulik, T.M.; Heger, M. Cholestasis Is Associated with Hepatic Microvascular Dysfunction and Aberrant Energy Metabolism Before and During Ischemia-Reperfusion. Antioxid. Redox Signal. 2012, 17, 1109–1123. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Nagino, M.; Nimura, Y. Mechanism of impaired hepatic regeneration in cholestatic liver. J. Hepato-Biliary-Pancreatic Surg. 2007, 14, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Wahab, M.A.; El Hanafy, E.; El Nakeeb, A.; Hamdy, E.; Atif, E.; Sultan, A.M. Postoperative Outcome after Major Liver Resection in Jaundiced Patients with Proximal Bile Duct Cancer without Preoperative Biliary Drainage. Dig. Surg. 2015, 32, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Olthof, P.B.; Coelen, R.J.; Wiggers, J.K.; Koerkamp, B.G.; Malago, M.; Hernandez-Alejandro, R.; Topp, S.A.; Vivarelli, M.; Aldrighetti, L.A.; Campos, R.R.; et al. High mortality after ALPPS for perihilar cholangiocarcinoma: Case-control analysis including the first series from the international ALPPS registry. HPB 2017, 19, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Oe, S.; Lemmer, E.R.; Conner, E.A.; Factor, V.M.; Levéen, P.; Larsson, J.; Karlsson, S.; Thorgeirsson, S.S. Intact Signaling by Transforming Growth Factor β Is Not Required for Termination of Liver Regeneration in Mice. Hepatology 2004, 40, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, K.L.; Trautwein, C.; Kubicka, S.; Rakemann, T.; Bahr, M.J.; Sedlaczek, N.; Schuppan, D.; Manns, M.P. Differential regulation of extracellular matrix synthesis during liver regeneration after partial hepatectomy in rats. Hepatology 1999, 30, 1159–1166. [Google Scholar] [CrossRef]
- Apte, U.; Gkretsi, V.; Bowen, W.C.; Mars, W.M.; Luo, J.-H.; Donthamsetty, S.; Orr, A.; Monga, S.P.S.; Wu, C.; Michalopoulos, G.K. Enhanced liver regeneration following changes induced by hepatocyte-specific genetic ablation of integrin-linked kinase. Hepatology 2009, 50, 844–851. [Google Scholar] [CrossRef] [Green Version]
- Yuan, B.; Dong, R.; Shi, D.; Zhou, Y.; Zhao, Y.; Miao, M.; Jiao, B. Down-regulation of miR-23b may contribute to activation of the TGF-β1/Smad3 signalling pathway during the termination stage of liver regeneration. FEBS Lett. 2011, 585, 927–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nygård, I.E.; Mortensen, K.E.; Hedegaard, J.; Conley, L.N.; Kalstad, T.; Bendixen, C.; Revhaug, A. The genetic regulation of the terminating phase of liver regeneration. Comp. Hepatol. 2012, 11, 15. [Google Scholar] [CrossRef] [Green Version]
- Bhave, V.S.; Mars, W.; Donthamsetty, S.; Zhang, X.; Tan, L.; Luo, J.; Bowen, W.C.; Michalopoulos, G.K. Regulation of Liver Growth by Glypican 3, CD81, Hedgehog, and Hhex. Am. J. Pathol. 2013, 183, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, J.; Hong, I.-H.; Lewis, K.; Iakova, P.; Breaux, M.; Jiang, Y.; Sullivan, E.; Jawanmardi, N.; Timchenko, L.; Timchenko, N.A. Cooperation of C/EBP family proteins and chromatin remodeling proteins is essential for termination of liver regeneration. Hepatology 2015, 61, 315–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Sola, G.; Uriarte, I.; Latasa, M.U.; Jimenez, M.; Barcena-Varela, M.; Santamaria, E.; Urtasun, R.; Rodriguez-Ortigosa, C.; Prieto, J.; Berraondo, P.; et al. Bile acids, FGF15/19 and liver regeneration: From mechanisms to clinical applications. Biochim. Biophys. Acta 2017, 1864, 1326–1334. [Google Scholar] [CrossRef]
- Merlen, G.; Ursic-Bedoya, J.; Jourdainne, V.; Kahale, N.; Glenisson, M.; Doignon, I.; Rainteau, M.; Tordjmann, T. Bile acids and their receptors during liver regeneration: “Dangerous protectors”. Mol. Asp. Med. 2017, 56, 25–33. [Google Scholar] [CrossRef]
- Olthof, P.B.; Huisman, F.; Schaap, F.G.; van Lienden, K.P.; Bennink, R.J.; van Golen, R.F.; Heger, M.; Verheij, J.; Jansen, P.L.; Damink, S.W.; et al. Effect of obeticholic acid on liver regeneration following portal vein embolization in an experimental model. Br. J. Surg. 2017, 104, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Trauner, M.; Nevens, F.; Shiffman, M.L.; Drenth, J.P.H.; Bowlus, C.L.; Vargas, V.; Andreone, P.; Hirschfield, G.M.; Pencek, R.; Malecha, E.S.; et al. Long-term efficacy and safety of obeticholic acid for patients with primary biliary cholangitis: 3-year results of an international open-label extension study. Lancet Gastroenterol. Hepatol. 2019, 4, 445–453. [Google Scholar] [CrossRef]
- Goldstein, J.; Levy, C. Novel and emerging therapies for cholestatic liver diseases. Liver Int. 2018, 38, 1520–1535. [Google Scholar] [CrossRef] [Green Version]
- Van Golen, R.F.; Olthof, P.B.; Lionarons, D.A.; Reiniers, M.J.; Alles, L.K.; Uz, Z.; de Haan, L.; Ergin, B.; de Waart, D.R.; Maas, A.; et al. FXR agonist obeticholic acid induces liver growth but exacerbates biliary injury in rats with obstructive cholestasis. Sci. Rep. 2018, 8, 16529. [Google Scholar] [CrossRef]
- Lionarons, D.A.; Heger, M.; van Golen, R.F.; Alles, L.K.; van der Mark, V.A.; Kloek, J.J.; de Waart, D.R.; Marsman, H.A.; Rusch, H.; Verheij, J.; et al. Simple steatosis sensitizes cholestatic rats to liver injury and dysregulates bile salt synthesis and transport. Sci. Rep. 2016, 6, 31829. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Pang, T.; Chiou, J.; Pleass, H.; Lam, V.; Hollands, M.; Johnston, E.; Richardson, A.; Yuen, L. Percutaneous vs. endoscopic pre-operative biliary drainage in hilar cholangiocarcinoma—A systematic review and meta-analysis. HPB 2016, 18, 400–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Y.; Tang, Q.; Xiong, X.; Li, F.; Ye, H.; Song, P.; Cheng, N. Preoperative biliary drainage versus direct surgery for perihilar cholangiocarcinoma: A retrospective study at a single center. Biosci. Trends 2017, 11, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Kloek, J.J.; Marsman, H.A.; van Vliet, A.K.; Gouma, D.J.; van Gulik, T.M. Biliary drainage attenuates postischemic reperfusion injury in the cholestatic rat liver. Surgery 2008, 144, 22–31. [Google Scholar] [CrossRef]
- Marsman, H.A.; de Graaf, W.; Heger, M.; van Golen, R.F.; Kate, F.J.; Bennink, R.; van Gulik, T.M. Hepatic regeneration and functional recovery following partial liver resection in an experimental model of hepatic steatosis treated with omega-3 fatty acids. Br. J. Surg. 2013, 100, 674–683. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, W.; Bennink, R.J.; Heger, M.; Maas, A.; de Bruin, K.; van Gulik, T.M. Quantitative Assessment of Hepatic Function During Liver Regeneration in a Standardized Rat Model. J. Nucl. Med. 2011, 52, 294–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Graaf, W.; Veteläinen, R.L.; de Bruin, K.; van Vliet, A.K.; van Gulik, T.M.; Bennink, R.J. 99mTc-GSA Scintigraphy with SPECT for Assessment of Hepatic Function and Functional Volume During Liver Regeneration in a Rat Model of Partial Hepatectomy. J. Nucl. Med. 2007, 49, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Graaf, W.; Heger, M.; Spruijt, O.; Maas, A.; de Bruin, K.; Hoekstra, R.; Bennink, R.J.; van Gulik, T.M. Quantitative Assessment of Liver Function after Ischemia-Reperfusion Injury and Partial Hepatectomy in Rats. J. Surg. Res. 2012, 172, 85–94. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, W.; Häusler, S.; Heger, M.; van Ginhoven, T.M.; van Cappellen, G.; Bennink, R.J.; Kullak-Ublick, G.A.; Hesselmann, R.; van Gulik, T.M.; Stieger, B. Transporters involved in the hepatic uptake of 99mTc-mebrofenin and indocyanine green. J. Hepatol. 2011, 54, 738–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heger, M.; de Graaf, W.; Bennink, R.J.; Beuers, U.; van Gulik, T.M. A Clinical Perspective on the Criteria for Liver Resection and the Use of Liver Function Tests. World J. Surg. 2010, 34, 868–869. [Google Scholar] [CrossRef] [Green Version]
- Rassam, F.; Olthof, P.B.; Bennink, R.J.; van Gulik, T.M. Current Modalities for the Assessment of Future Remnant Liver Function. Visc. Med. 2017, 33, 442–448. [Google Scholar] [CrossRef]
- Olthof, P.B.; van Golen, R.F.; Reiniers, M.J.; Kos, M.; Alles, L.K.; Maas, M.A.; Verheij, J.; van Gulik, T.M.; Heger, M. IL-23 and IL-17A are not involved in hepatic/ischemia reperfusion injury in mouse and man. J. Clin. Transl. Res. 2015, 1, 180–189. [Google Scholar]
- Sakka, S.G.; van Hout, N. Relation between indocyanine green (ICG) plasma disappearance rate and ICG blood clearance in critically ill patients. Intensiv. Care Med. 2006, 32, 766–769. [Google Scholar] [CrossRef]
- Olthof, P.B.; van Golen, R.F.; Meijer, B.; van Beek, A.A.; Bennink, R.J.; Verheij, J.; van Gulik, T.M.; Heger, M. Warm ischemia time-dependent variation in liver damage, inflammation, and function in hepatic ischemia/reperfusion injury. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 375–385. [Google Scholar] [CrossRef]
- Ruijter, J.M.; Ramakers, C.; Hoogaars, W.M.H.; Karlen, Y.; Bakker, O.; Hoff, M.J.B.V.D.; Moorman, A.F.M. Amplification efficiency: Linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009, 37, e45. [Google Scholar] [CrossRef] [Green Version]
- Van Golen, R.F.; Olthof, P.B.; de Haan, L.R.; Coelen, R.J.; Pechlivanis, A.; de Keijzer, M.J.; Weijer, R.; de Waart, D.R.; van Kuilenburg, A.B.; Roelofsen, J.; et al. The pathophysiology of human obstructive cholestasis is mimicked in cholestatic Gold Syrian hamsters. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Lorbek, G.; Lewinska, M.; Rozman, D. Cytochrome P450s in the synthesis of cholesterol and bile acids - from mouse models to human diseases. FEBS J. 2011, 279, 1516–1533. [Google Scholar] [CrossRef]
- Inagaki, T.; Choi, M.; Moschetta, A.; Peng, L.; Cummins, C.L.; McDonald, J.G.; Luo, G.; Jones, S.A.; Goodwin, B.; Richardson, J.A.; et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005, 2, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Rassam, F.; Olthof, P.B.; Richardson, H.; van Gulik, T.M.; Bennink, R.J. Practical guidelines for the use of technetium-99m mebrofenin hepatobiliary scintigraphy in the quantitative assessment of liver function. Nucl. Med. Commun. 2019, 40, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L. Bile Acid Metabolism and Signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myant, N.B.; Mitropoulos, K.A. Cholesterol 7 alpha-hydroxylase. J. Lipid Res. 1977, 18, 135–153. [Google Scholar] [CrossRef]
- Ijssennagger, N.; Janssen, A.W.; Milona, A.; Pittol, J.M.R.; Hollman, D.A.; Mokry, M.; Betzel, B.; Berends, F.J.; Janssen, I.M.; van Mil, S.W.; et al. Gene expression profiling in human precision cut liver slices in response to the FXR agonist obeticholic acid. J. Hepatol. 2016, 64, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Fickert, P.; Zollner, G.; Fuchsbichler, A.; Silbert, D.; Tsybrovskyy, O.; Zatloukal, K.; Guo, G.L.; Schuetz, J.D.; Gonzalez, F.J.; et al. Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver injury in bile duct-ligated mice. Gastroenterology 2003, 125, 825–838. [Google Scholar] [CrossRef]
- Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.-U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut Microbiota Regulates Bile Acid Metabolism by Reducing the Levels of Tauro-beta-muricholic Acid, a Naturally Occurring FXR Antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Chen, I.; Cassaro, S. Physiology, Bile Acids. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2020. [Google Scholar]
- Bile Acids. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. 25 September 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548626/ (accessed on 28 December 2020).
- Cai, J.-S.; Chen, J.-H. The Mechanism of Enterohepatic Circulation in the Formation of Gallstone Disease. J. Membr. Biol. 2014, 247, 1067–1082. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.-D.; Wang, Y.-D.; Zhang, L.; Shiah, S.; Wang, M.; Yang, F.; Yu, D.; Forman, B.M.; Huang, W. Farnesoid X receptor alleviates age-related proliferation defects in regenerating mouse livers by activating forkhead box m1b transcription. Hepatology 2009, 51, 953–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaap, F.G.; van der Gaag, N.A.; Gouma, D.J.; Jansen, P.L.M. High expression of the bile salt-homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology 2008, 49, 1228–1235. [Google Scholar] [CrossRef]
- Russell, D.W. The Enzymes, Regulation, and Genetics of Bile Acid Synthesis. Annu. Rev. Biochem. 2003, 72, 137–174. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Jiang, C.; Cheng, J.; Krausz, K.W.; Li, T.; Ferrell, J.M.; Gonzalez, F.J.; Chiang, J.Y. Bile acid signaling in lipid metabolism: Metabolomic and lipidomic analysis of lipid and bile acid markers linked to anti-obesity and anti-diabetes in mice. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 19–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holt, J.A.; Luo, G.; Billin, A.N.; Bisi, J.; McNeill, Y.Y.; Kozarsky, K.F.; Donahee, M.H.; Wang, D.Y.; Mansfield, T.A.; Kliewer, S.A.; et al. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003, 17, 1581–1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodrich, D.W.; Wang, N.P.; Qian, Y.-W.; Lee, E.Y.-H.; Lee, W.-H. The retinoblastoma gene product regulates progression through the G1 phase of the cell cycle. Cell 1991, 67, 293–302. [Google Scholar] [CrossRef]
- Weintraub, S.J.; Prater, C.A.; Dean, D.C. Retinoblastoma protein switches the E2F site from positive to negative element. Nat. Cell Biol. 1992, 358, 259–261. [Google Scholar] [CrossRef]
- New drug: Obeticholic acid for primary biliary cholangitis. Aust. Prescr. 2020, 43, 174–175. [CrossRef]
- Nakamura, T.; Tomita, Y.; Hirai, R.; Yamaoka, K.; Kaji, K.; Ichihara, A. Inhibitory effect of transforming growth factor-beta on DNA synthesis of adult rat hepatocytes in primary culture. Biochem. Biophys. Res. Commun. 1985, 133, 1042–1050. [Google Scholar] [CrossRef]
- Ananthanarayanan, M.; Balasubramanian, N.; Makishima, M.; Mangelsdorf, D.J.; Suchy, F.J. Human Bile Salt Export Pump Promoter Is Transactivated by the Farnesoid X Receptor/Bile Acid Receptor. J. Biol. Chem. 2001, 276, 28857–28865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyer, J.L.; Trauner, M.; Mennone, A.; Soroka, C.J.; Cai, S.-Y.; Moustafa, T.; Zollner, G.; Lee, J.Y.; Ballatori, N. Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTα-OSTβ in cholestasis in humans and rodents. Am. J. Physiol. Liver Physiol. 2006, 290, G1124–G1130. [Google Scholar] [CrossRef]
- Kast, H.R.; Goodwin, B.; Tarr, P.T.; Jones, S.A.; Anisfeld, A.M.; Stoltz, C.M.; Tontonoz, P.; A Kliewer, S.; Willson, T.M.; Edwards, P.A. Regulation of Multidrug Resistance-associated Protein 2 (ABCC2) by the Nuclear Receptors Pregnane X Receptor, Farnesoid X-activated Receptor, and Constitutive Androstane Receptor. J. Biol. Chem. 2002, 277, 2908–2915. [Google Scholar] [CrossRef] [Green Version]
- Miao, J.; Xiao, Z.; Kanamaluru, D.; Min, G.; Yau, P.M.; Veenstra, T.D.; Ellis, E.; Strom, S.; Suino-Powell, K.; Xu, H.E.; et al. Bile acid signaling pathways increase stability of Small Heterodimer Partner (SHP) by inhibiting ubiquitin-proteasomal degradation. Genes Dev. 2009, 23, 986–996. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-L.; Liu, Y.-J.; Chen, H.-L.; Wu, S.-H.; Ni, Y.-H.; Ho, M.-C.; Lai, H.-S.; Hsu, W.-M.; Hsu, H.-Y.; Tseng, H.-C.; et al. Expression of Hepatocyte Transporters and Nuclear Receptors in Children with Early and Late-Stage Biliary Atresia. Pediatr. Res. 2008, 63, 667–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keitel, V.; Burdelski, M.; Warskulat, U.; Kühlkamp, T.; Keppler, D.; Häussinger, D.; Kubitz, R. Expression and localization of hepatobiliary transport proteins in progressive familial intrahepatic cholestasis. Hepatology 2005, 41, 1160–1172. [Google Scholar] [CrossRef]
- Le Vee, M.; Gripon, P.; Stieger, B.; Fardel, O. Down-Regulation of Organic Anion Transporter Expression in Human Hepatocytes Exposed to the Proinflammatory Cytokine Interleukin 1β. Drug Metab. Dispos. 2007, 36, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Meier, P.J.; Stieger, B. Bile Salt Transporters. Annu. Rev. Physiol. 2002, 64, 635–661. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.A.; Lan, T.; Rao, A. Bile acid transporters. J. Lipid Res. 2009, 50, 2340–2357. [Google Scholar] [CrossRef] [Green Version]
- Landrier, J.-F.; Eloranta, J.J.; Vavricka, S.R.; Kullak-Ublick, G.A. The nuclear receptor for bile acids, FXR, transactivates human organic solute transporter-α and -β genes. Am. J. Physiol. Liver Physiol. 2006, 290, G476–G485. [Google Scholar] [CrossRef] [Green Version]
- Gartung, C.; Matern, S. Molecular regulation of sinusoidal liver bile acid transporters during cholestasis. Yale J. Boil. Med. 1997, 70, 355–363. [Google Scholar]
- Hagenbuch, B.; Stieger, B.; Foguet, M.; Lubbert, H.; Meier, P.J. Functional expression cloning and characterization of the hepatocyte Na+/bile acid cotransport system. Proc. Natl. Acad. Sci. USA 1991, 88, 10629–10633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stieger, B.; Hagenbuch, B.; Landmann, L.; Höchli, M.; Schroeder, A.; Meier, P.J. In situ localization of the hepatocytic na+/taurocholate cotransporting polypeptide in rat liver. Gastroenterology 1994, 107, 1781–1787. [Google Scholar] [CrossRef]
- Vaz, F.M.; Paulusma, C.C.; Huidekoper, H.; de Ru, M.; Lim, C.; Koster, J.; Ho-Mok, K.; Bootsma, A.H.; Groen, A.K.; Schaap, F.G.; et al. Sodium taurocholate cotransporting polypeptide (SLC10A1) deficiency: Conjugated hypercholanemia without a clear clinical phenotype. Hepatology 2014, 61, 260–267. [Google Scholar] [CrossRef]
- Geier, A.; Dietrich, C.G.; Voigt, S.; Kim, S.; Gerloff, T.; Kullak-Ublick, G.A.; Lorenzen, J.; Matern, S.; Gartung, C. Effects of proinflammatory cytokines on rat organic anion transporters during toxic liver injury and cholestasis. Hepatology 2003, 38, 345–354. [Google Scholar] [CrossRef] [Green Version]
- Sturm, E.; Havinga, R.; Baller, J.F.; Wolters, H.; van Rooijen, N.; Kamps, J.A.; Verkade, H.J.; Karpen, S.J.; Kuipers, F. Kupffer cell depletion with liposomal clodronate prevents suppression of Ntcp expression in endotoxin-treated rats. J. Hepatol. 2005, 42, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Frisch, K.; Keiding, S. Use of Indocyanine Green in Hepatology. Adv. Res. Gastroenterol. Hepatol. 2020, 16. [Google Scholar] [CrossRef]








| Parameter | PHC (n = 23) | CRLM (n = 51) | p-Value |
|---|---|---|---|
| Age, median (IQR) | 67 (60–71) | 65 (58–69) | 0.245 |
| Male sex, n (%) | 16 (70) | 34 (67) | 0.807 |
| Days between PVE and CT, median (IQR) | 23 (21–26) | 22 (21–25) | 0.777 |
| Days between PVE and HBS, median (IQR) | 22 (21–24) | 22 (21–23) | 0.362 |
| Increase liver volume, %, median (IQR) | 35 (15–62) | 36 (27–61) | 0.392 |
| Increase liver function, %, median (IQR) | 57 (30–89) | 54 (35–92) | 0.784 |
| AST, U/L, median (IQR) | 69 (36–80) | 37 (27–56) | 0.133 |
| ALT, U/L, median (IQR) | 73 (52–114) | 28 (23–44) | 0.012 * |
| ALP, U/L, median (IQR) | 312 (166–620) | 121 (77–154) | 0.00 ** |
| gGT, U/L, median (IQR) | 479 (252–923) | 37 (29–108) | 0.00 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Haan, L.R.; Verheij, J.; van Golen, R.F.; Horneffer-van der Sluis, V.; Lewis, M.R.; Beuers, U.H.W.; van Gulik, T.M.; Olde Damink, S.W.M.; Schaap, F.G.; Heger, M.; et al. Unaltered Liver Regeneration in Post-Cholestatic Rats Treated with the FXR Agonist Obeticholic Acid. Biomolecules 2021, 11, 260. https://doi.org/10.3390/biom11020260
de Haan LR, Verheij J, van Golen RF, Horneffer-van der Sluis V, Lewis MR, Beuers UHW, van Gulik TM, Olde Damink SWM, Schaap FG, Heger M, et al. Unaltered Liver Regeneration in Post-Cholestatic Rats Treated with the FXR Agonist Obeticholic Acid. Biomolecules. 2021; 11(2):260. https://doi.org/10.3390/biom11020260
Chicago/Turabian Stylede Haan, Lianne R., Joanne Verheij, Rowan F. van Golen, Verena Horneffer-van der Sluis, Matthew R. Lewis, Ulrich H. W. Beuers, Thomas M. van Gulik, Steven W. M. Olde Damink, Frank G. Schaap, Michal Heger, and et al. 2021. "Unaltered Liver Regeneration in Post-Cholestatic Rats Treated with the FXR Agonist Obeticholic Acid" Biomolecules 11, no. 2: 260. https://doi.org/10.3390/biom11020260
APA Stylede Haan, L. R., Verheij, J., van Golen, R. F., Horneffer-van der Sluis, V., Lewis, M. R., Beuers, U. H. W., van Gulik, T. M., Olde Damink, S. W. M., Schaap, F. G., Heger, M., & Olthof, P. B. (2021). Unaltered Liver Regeneration in Post-Cholestatic Rats Treated with the FXR Agonist Obeticholic Acid. Biomolecules, 11(2), 260. https://doi.org/10.3390/biom11020260