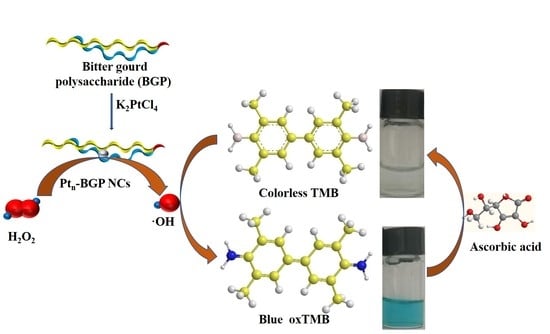
Biocompatible Platinum Nanoclusters Prepared Using Bitter Gourd Polysaccharide for Colorimetric Detection of Ascorbic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of Pt-BGP NCs
2.3. Determination of Ascorbic Acid Concentration
2.4. Biocompatibility of Pt-BGP NCs
3. Results
3.1. Characterization of GBP
3.2. Characterization of Pt -BGP NCs
3.3. Peroxidase-Like Activity and Catalytic Mechanism
3.4. Determination of Ascorbic Acid Concentration
3.5. Biocompatibility
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, S.; Chae, J.S.; Shin, H.; Shin, Y.; Song, H.; Kim, Y.; Yoo, B.C. Hormetic dose response to L-ascorbic acid as an anti-cancer drug in colorectal cancer cell lines according to SVCT-2 expression. Sci. Rep. 2018, 8, 11372. [Google Scholar] [CrossRef] [Green Version]
- Siripongpreda, T.; Somchob, B.; Rodthongkum, N.; Hoven, V.P. Bacterial cellulose-based re-swellable hydrogel: Facile preparation and its potential application as colorimetric sensor of sweat pH and glucose. Carbohydr. Polym. 2021, 256, 117506. [Google Scholar] [CrossRef]
- Soares, A.; Goncalves, L.M.O.; Ferreira, R.D.S.; de Souza, J.M.; Fangueiro, R. Immobilization of papain enzyme on a hybrid support containing zinc oxide nanoparticles and chitosan for clinical applications. Carbohydr. Polym. 2020, 243, 116498. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, M.; Liu, Y.; Meng, X.; Ye, Y.; Song, X.; Liang, Z. Novel D-π-A conjugated microporous polymer as visible light-driven oxidase mimic for efficient colorimetric detection of glutathione. Sens. Actuator B Chem. 2021, 326, 128808. [Google Scholar] [CrossRef]
- Chen, Y.; Jiao, L.; Yan, H.; Xu, W.; Wu, Y.; Wang, H.; Gu, W. Hierarchically porous S/N codoped carbon nanozymes with enhanced peroxidase-like activity for total tntioxidant capacity biosensing. Anal. Chem. 2020, 92, 13518–13524. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Zhao, X.; Yao, X.; Chen, W.; Liu, Z.; Gao, L.; Fan, G.; Zhu, X.; Zhang, X. 3,4:9,10-perylene tetracarboxylic acid-modified zinc ferrite with the enhanced peroxidase activity for sensing of ascorbic acid. Colloid Surf. A Physicochem. Eng. Asp. 2020, 586, 124250. [Google Scholar] [CrossRef]
- Wang, L.; Clardy, A.; Hui, D.; Gao, A.; Wu, Y. Antioxidant and antidiabetic properties of Chinese and Indian bitter melons (Momordica charantia L.). Food Biosci. 2019, 29, 73–80. [Google Scholar] [CrossRef]
- Panda, B.C.; Mondal, S.; Devi, K.S.; Maiti, T.K.; Khatua, S.; Acharya, K.; Islam, S.S. Pectic polysaccharide from the green fruits of Momordica charantia (Karela): Structural characterization and study of immunoenhancing and antioxidant properties. Carbohydr. Res. 2015, 401, 24–31. [Google Scholar] [CrossRef]
- Dong, L.; Li, R.; Wang, L.; Lan, X.; Sun, H.; Zhao, Y.; Wang, L. Green synthesis of platinum nanoclusters using lentinan for sensitively colorimetric detection of glucose. Int. J. Biol. Macromol. 2021, 172, 289–298. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Singh, R. Green biomimetic synthesis of Ag-TiO2 nanocomposite using origanum majorana leaf extract under sonication and their biological activities. Bioresources 2021, 8, 1009. [Google Scholar]
- Yan, J.K.; Wu, L.X.; Qiao, Z.R.; Cai, W.D.; Ma, H. Effect of different drying methods on the product quality and bioactive polysaccharides of bitter gourd (Momordica charantia L.) slices. Food Chem. 2019, 271, 588–596. [Google Scholar] [CrossRef]
- Yan, J.K.; Yu, Y.B.; Wang, C.; Cai, W.D.; Wu, L.X.; Yang, Y. Production, physicochemical characteristics, and in vitro biological activities of polysaccharides obtained from fresh bitter gourd (Momordica charantia L.) via room temperature extraction techniques. Food Chem. 2021, 337, 127798. [Google Scholar] [CrossRef]
- Cui, T.; Li, S.; Chen, S.; Liang, Y.; Sun, H.; Wang, L. “Stealth” dendrimers with encapsulation of indocyanine green for photothermal and photodynamic therapy of cancer. Int. J. Pharm. 2021, 600, 120502. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, G. Antioxidant activities of phosphorylated pumpkin polysaccharide. Int. J. Biol. Macromol. 2019, 125, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhang, H.; Zhang, J.; Wen, C.; Ma, H.; Duan, Y.; He, Y. Preparation, characterization and bioactivity of polysaccharide fractions from Sagittaria sagittifolia L. Carbohydr. Polym. 2020, 229, 115355. [Google Scholar] [CrossRef] [PubMed]
- Dobrucka, R. Biofabrication of platinum nanoparticles using Fumariae herba extract and their catalytic properties. Saudi J. Biol. Sci. 2019, 26, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, F.; Long, R.; Huang, G. The antioxidant activities in vivo of bitter gourd polysaccharide. Int. J. Biol. Macromol. 2020, 145, 141–144. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Han, Q.; Qi, C.; Wang, C.; Yang, R. Facile synthesis of IrO2/rGO nanocomposites with high peroxidase-like activity for sensitive colorimetric detection of low weight biothiols. Talanta 2019, 203, 227–234. [Google Scholar] [CrossRef]
- Dong, W.; Huang, Y. CeO2/C nanowire derived from a cerium (III) based organic framework as a peroxidase mimic for colorimetric sensing of hydrogen peroxide and for enzymatic sensing of glucose. Mikrochim. Acta 2019, 187, 11. [Google Scholar] [CrossRef]
- Kora, A.J.; Rastogi, L. Peroxidase activity of biogenic platinum nanoparticles: A colorimetric probe towards selective detection of mercuric ions in water samples. Sens. Actuator B Chem. 2018, 254, 690–700. [Google Scholar] [CrossRef]
- Fan, L.; Ji, X.; Lin, G.; Liu, K.; Chen, S.; Ma, G.; Xue, W.; Zhang, X.; Wang, L. Green synthesis of stable platinum nanoclusters with enhanced peroxidase-like activity for sensitive detection of glucose and glutathione. Microchem. J. 2021, 166, 106202. [Google Scholar] [CrossRef]
- Shi, W.; Shuang, E.; Wang, M.M.; Li, T.Z.; Yang, T.; Liu, S.R. Facile synthesis of metal-organic framework-derived SiW12@Co3O4 and its peroxidase-like activity in colorimetric assay. Analyst 2019, 144, 5455–5461. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wu, Z.; Liu, G.; Lu, H.; Gao, Y.; Liu, F.; Wang, C.; Cui, J.; Lu, G. High-activity Mo, S co-doped carbon quantum dot nanozyme-based cascade colorimetric biosensor for sensitive detection of cholesterol. J. Mater. Chem. B 2019, 7, 7042–7051. [Google Scholar] [CrossRef]
- Li, X.; Sun, L.; Yang, X.; Zhou, K.; Zhang, G.; Tong, Z.; Wang, C.; Sha, J. Enhancing the colorimetric detection of H2O2 and ascorbic acid on polypyrrole coated fluconazole-functionalized POMOFs. Analyst 2019, 144, 3347–3356. [Google Scholar] [CrossRef]
- Alizadeh, N.; Ghasemi, F.; Salimi, A.; Hallaj, R.; Fathi, F.; Soleimani, F. Polymer nanocomposite film for dual colorimetric and fluorescent ascorbic acid detection integrated single-cell bioimaging with droplet microfluidic platform. Dyes Pigment. 2020, 173, 107875. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, J.; Li, N.; You, Z.; Feng, Z.; Natarajan, V. Co3O4@β-cyclodextrin with synergistic peroxidase-mimicking performance as a signal magnification approach for colorimetric determination of ascorbic acid. Sens. Actuator B Chem. 2020, 303, 127106. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, C.; Yang, X.; Cheng, X.; Elzatahry, A.A.; Alghamdi, A.; Su, J.; Deng, Y. Hollow mesoporous carbon nanospheres loaded with Pt nanoparticles for colorimetric detection of ascorbic acid and glucose. ACS Appl. Nano Mater. 2020, 3, 4586–4598. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G. Extraction, derivatization and antioxidant activity of bitter gourd polysaccharide. Int. J. Biol. Macromol. 2019, 141, 14–20. [Google Scholar] [CrossRef]
- Eramabadi, P.; Masoudi, M.; Makhdoumi, A.; Mashreghi, M. Microbial cell lysate supernatant (CLS) alteration impact on platinum nanoparticles fabrication, characterization, antioxidant and antibacterial activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111292. [Google Scholar] [CrossRef]
- Lian, J.; Liu, P.; Li, X.; Bian, B.; Zhang, X.; Liu, Z.; Liu, Q. Multi-layer CeO2-wrapped Ag2S microspheres with enhanced peroxidase-like activity for sensitive detection of dopamine. Colloid Surf. A Physicochem. Eng. Asp. 2019, 565, 1–7. [Google Scholar] [CrossRef]
- Yang, C.; Wang, M.; Zhou, J.; Chi, Q. Bio-synthesis of peppermint leaf extract polyphenols capped nano-platinum and their in-vitro cytotoxicity towards colon cancer cell lines (HCT 116). Mater Sci. Eng. C Mater. Biol. Appl. 2017, 77, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.; Namratha, K.; Thakur, M.S.; Byrappa, K. Biocompatibility assessment and photocatalytic activity of bio-hydrothermal synthesis of ZnO nanoparticles by thymus vulgaris leaf extract. Mater. Res. Bull. 2019, 109, 49–59. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Zhao, Y.; Zhang, L.; He, M.; Lin, W.; Sun, H.; Liu, Z.; Hu, J.; Wang, L. Biocompatible Platinum Nanoclusters Prepared Using Bitter Gourd Polysaccharide for Colorimetric Detection of Ascorbic Acid. Biomolecules 2021, 11, 647. https://doi.org/10.3390/biom11050647
Liu K, Zhao Y, Zhang L, He M, Lin W, Sun H, Liu Z, Hu J, Wang L. Biocompatible Platinum Nanoclusters Prepared Using Bitter Gourd Polysaccharide for Colorimetric Detection of Ascorbic Acid. Biomolecules. 2021; 11(5):647. https://doi.org/10.3390/biom11050647
Chicago/Turabian StyleLiu, Kai, Yu Zhao, Lu Zhang, Mengmeng He, Weifeng Lin, Haotian Sun, Zhiwei Liu, Jie Hu, and Longgang Wang. 2021. "Biocompatible Platinum Nanoclusters Prepared Using Bitter Gourd Polysaccharide for Colorimetric Detection of Ascorbic Acid" Biomolecules 11, no. 5: 647. https://doi.org/10.3390/biom11050647
APA StyleLiu, K., Zhao, Y., Zhang, L., He, M., Lin, W., Sun, H., Liu, Z., Hu, J., & Wang, L. (2021). Biocompatible Platinum Nanoclusters Prepared Using Bitter Gourd Polysaccharide for Colorimetric Detection of Ascorbic Acid. Biomolecules, 11(5), 647. https://doi.org/10.3390/biom11050647