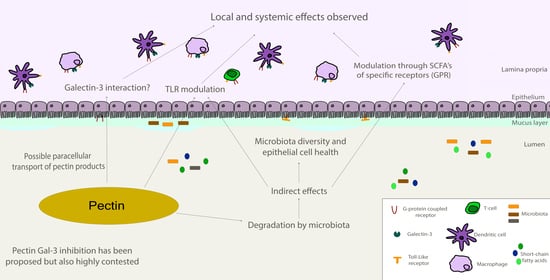
The Complex Biological Effects of Pectin: Galectin-3 Targeting as Potential Human Health Improvement?
Abstract
:1. Introduction
2. Basic Pectin Molecular Aspects
2.1. Pectin Molecular Weight
2.2. Monosaccharides, Backbone, and Side Chains
2.3. Esterification Degree
2.4. Rheological Properties
2.5. Food Source
3. Gal-3 Binding Sites and Pectin Interactions
4. Pectin and Gal-3 Controversies
5. Pectin as Dietary Fiber: Some of the Gal-3 Independent Beneficial Effects to Human Health
6. Should Gal-3 Inhibition Be the Main Biological Effect Expected from Pectin?
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-related gelling of pectins and linking with other natural compounds: A review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, E.G.; Belshaw, N.J.; Waldron, K.W.; Morris, V.J. Pectin—An emerging new bioactive food polysaccharide. Trends Food Sci. Technol. 2012, 24, 64–73. [Google Scholar] [CrossRef]
- Cui, L.; Wang, J.; Huang, R.; Tan, Y.; Zhang, F.; Zhou, Y.; Sun, L. Analysis of pectin from Panax ginseng flower buds and their binding activities to galectin-3. Int. J. Biol. Macromol. 2019, 128, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Fabi, J.P.; Seymour, G.B.; Graham, N.S.; Broadley, M.R.; May, S.T.; Lajolo, F.M.; Cordenunsi, B.R.; Oliveira do Nascimento, J.R. Analysis of ripening-related gene expression in papaya using an Arabidopsis-based microarray. BMC Plant Biol. 2012, 12, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabi, J.P.; Broetto, S.G.; da Silva, S.L.G.L.; Zhong, S.; Lajolo, F.M.; do Nascimento, J.R.O. Analysis of papaya cell wall-related genes during fruit ripening indicates a central role of polygalacturonases during pulp softening. PLoS ONE 2014, 9, e105685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Freitas Pedrosa, L.; Lopes, R.G.; Fabi, J.P. The acid and neutral fractions of pectins isolated from ripe and overripe papayas differentially affect galectin-3 inhibition and colon cancer cell growth. Int. J. Biol. Macromol. 2020, 164, 2681–2690. [Google Scholar] [CrossRef] [PubMed]
- Do Prado, S.B.R.; Ferreira, G.F.; Harazono, Y.; Shiga, T.M.; Raz, A.; Carpita, N.C.; Fabi, J.P. Ripening-induced chemical modifications of papaya pectin inhibit cancer cell proliferation. Sci. Rep. 2017, 7, 16564. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Cummings, R.D.; Aebi, M.; Packer, N.H.; Seeberger, P.H.; Esko, J.D.; Stanley, P.; Hart, G.; Darvill, A.; Kinoshita, T.; et al. Symbol nomenclature for graphical representations of glycans. Glycobiology 2015, 25, 1323–1324. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Xu, C.; Huang, R.; Song, J.; Li, D.; Xia, M. Butyrate from pectin fermentation inhibits intestinal cholesterol absorption and attenuates atherosclerosis in apolipoprotein E-deficient mice. J. Nutr. Biochem. 2018, 56, 175–182. [Google Scholar] [CrossRef]
- Li, W.; Zhang, K.; Yang, H. Pectin Alleviates High Fat (Lard) Diet-Induced Nonalcoholic Fatty Liver Disease in Mice: Possible Role of Short-Chain Fatty Acids and Gut Microbiota Regulated by Pectin. J. Agric. Food Chem. 2018, 66, 8015–8025. [Google Scholar] [CrossRef] [PubMed]
- Brouns, F.; Theuwissen, E.; Adam, A.; Bell, M.; Berger, A.; Mensink, R.P. Cholesterol-lowering properties of different pectin types in mildly hyper-cholesterolemic men and women. Eur. J. Clin. Nutr. 2012, 66, 591–599. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Dong, M.; Yang, Z.; Pan, S. Anti-diabetic effect of citrus pectin in diabetic rats and potential mechanism via PI3K/Akt signaling pathway. Int. J. Biol. Macromol. 2016, 89, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Fotschki, B.; Jurgoński, A.; Juśkiewicz, J.; Kołodziejczyk, K.; Sójka, M. Effects of dietary addition of a low-pectin apple fibre preparation on rats. Pol. J. Food Nutr. Sci. 2014, 64, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Kunzmann, A.T.; Coleman, H.G.; Huang, W.-Y.; Kitahara, C.M.; Cantwell, M.M.; Berndt, S.I. Dietary fiber intake and risk of colorectal cancer and incident and recurrent adenoma in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am. J. Clin. Nutr. 2015, 102, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Tang, J.-W.; Owusu, L.; Sun, M.-Z.; Wu, J.; Zhang, J. Galectin-3 in cancer. Clin. Chim. Acta 2014, 431, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Sciacchitano, S.; Lavra, L.; Morgante, A.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Ricci, A. Galectin-3: One molecule for an alphabet of diseases, from A to Z. Int. J. Mol. Sci. 2018, 19, 379. [Google Scholar] [CrossRef] [Green Version]
- Fortuna-Costa, A.; Gomes, A.M.; Kozlowski, E.O.; Stelling, M.P.; Pavão, M.S.G. Extracellular galectin-3 in tumor progression and metastasis. Front. Oncol. 2014, 4. [Google Scholar] [CrossRef] [Green Version]
- Suthahar, N.; Meijers, W.C.; Silljé, H.H.W.; Ho, J.E.; Liu, F.-T.; de Boer, R.A. Galectin-3 activation and inhibition in heart failure and cardiovascular disease: An update. Theranostics 2018, 8, 593–609. [Google Scholar] [CrossRef]
- Filipová, M.; Bojarová, P.; Rodrigues Tavares, M.; Bumba, L.; Elling, L.; Chytil, P.; Gunár, K.; Křen, V.; Etrych, T.; Janoušková, O. Glycopolymers for Efficient Inhibition of Galectin-3: In Vitro Proof of Efficacy Using Suppression of T Lymphocyte Apoptosis and Tumor Cell Migration. Biomacromolecules 2020, 21, 3122–3133. [Google Scholar] [CrossRef]
- Jia, J.; Claude-Taupin, A.; Gu, Y.; Choi, S.W.; Peters, R.; Bissa, B.; Mudd, M.H.; Allers, L.; Pallikkuth, S.; Lidke, K.A.; et al. Galectin-3 Coordinates a Cellular System for Lysosomal Repair and Removal. Dev. Cell 2020, 52, 69–87.e8. [Google Scholar] [CrossRef]
- Maxwell, E.G.; Colquhoun, I.J.; Chau, H.K.; Hotchkiss, A.T.; Waldron, K.W.; Morris, V.J.; Belshaw, N.J. Rhamnogalacturonan i containing homogalacturonan inhibits colon cancer cell proliferation by decreasing ICAM1 expression. Carbohydr. Polym. 2015, 132, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-L.; Huang, E.-Y.; Jhu, E.-W.; Huang, Y.-H.; Su, W.-H.; Chuang, P.-C.; Yang, K.D. Overexpression of galectin-3 enhances migration of colon cancer cells related to activation of the K-Ras-Raf-Erk1/2 pathway. J. Gastroenterol. 2013, 48, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ji, B.; Ramachandran, V.; Wang, H.; Hafley, M.; Logsdon, C.; Bresalier, R.S. Overexpressed galectin-3 in pancreatic cancer induces cell proliferation and invasion by binding ras and activating ras signaling. PLoS ONE 2012, 7, e42699. [Google Scholar] [CrossRef] [Green Version]
- Margadant, C.; Van Den Bout, I.; Van Boxtel, A.L.; Thijssen, V.L.; Sonnenberg, A. Epigenetic regulation of galectin-3 expression by β1 integrins promotes cell adhesion and migration. J. Biol. Chem. 2012, 287, 44684–44693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Guo, H.; Geng, J.; Zheng, X.; Wei, H.; Sun, R.; Tian, Z. Tumor-released galectin-3, a soluble inhibitory ligand of human NKp30, plays an important role in tumor escape from NK cell attack. J. Biol. Chem. 2014, 289, 33311–33319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voss, J.J.L.P.; Ford, C.A.; Petrova, S.; Melville, L.; Paterson, M.; Pound, J.D.; Holland, P.; Giotti, B.; Freeman, T.C.; Gregory, C.D. Modulation of macrophage antitumor potential by apoptotic lymphoma cells. Cell Death Differ. 2017, 24, 971–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, H.; Liu, L.; Zhao, Z.; Zhang, Z.; Guan, Y.; Cheng, H.; Zhou, Y.; Tai, G. The N-terminal tail coordinates with carbohydrate recognition domain to mediate galectin-3 induced apoptosis in T cells. Oncotarget 2017, 8, 49824–49838. [Google Scholar] [CrossRef]
- Freichel, T.; Heine, V.; Laaf, D.; Mackintosh, E.E.; Sarafova, S.; Elling, L.; Snyder, N.L.; Hartmann, L. Sequence-Defined Heteromultivalent Precision Glycomacromolecules Bearing Sulfonated/Sulfated Nonglycosidic Moieties Preferentially Bind Galectin-3 and Delay Wound Healing of a Galectin-3 Positive Tumor Cell Line in an In Vitro Wound Scratch Assay. Macromol. Biosci. 2020, 20, 2000163. [Google Scholar] [CrossRef]
- Laaf, D.; Bojarová, P.; Elling, L.; Křen, V. Galectin—Carbohydrate Interactions in Biomedicine and Biotechnology. Trends Biotechnol. 2019, 37, 402–415. [Google Scholar] [CrossRef]
- Rajput, V.K.; MacKinnon, A.; Mandal, S.; Collins, P.; Blanchard, H.; Leffler, H.; Sethi, T.; Schambye, H.; Mukhopadhyay, B.; Nilsson, U.J. A Selective Galactose-Coumarin-Derived Galectin-3 Inhibitor Demonstrates Involvement of Galectin-3-glycan Interactions in a Pulmonary Fibrosis Model. J. Med. Chem. 2016, 59, 8141–8147. [Google Scholar] [CrossRef] [PubMed]
- Do Prado, S.B.R.; Santos, G.R.C.; Mourão, P.A.S.; Fabi, J.P. Chelate-soluble pectin fraction from papaya pulp interacts with galectin-3 and inhibits colon cancer cell proliferation. Int. J. Biol. Macromol. 2019, 126, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zheng, J.; Hu, W.; Zheng, X.; He, Q.; Linhardt, R.J.; Ye, X.; Chen, S. Structure-activity relationship of Citrus segment membrane RG-I pectin against Galectin-3: The galactan is not the only important factor. Carbohydr. Polym. 2020, 245, 116526. [Google Scholar] [CrossRef]
- Zhang, T.; Lan, Y.; Zheng, Y.; Liu, F.; Zhao, D.; Mayo, K.H.; Zhou, Y.; Tai, G. Identification of the bioactive components from pH-modified citrus pectin and their inhibitory effects on galectin-3 function. Food Hydrocoll. 2016, 58, 113–119. [Google Scholar] [CrossRef]
- Do Nascimento Oliveira, A.; de Almeida Paula, D.; de Oliveira, E.B.; Saraiva, S.H.; Stringheta, P.C.; Ramos, A.M. Optimization of pectin extraction from Ubá mango peel through surface response methodology. Int. J. Biol. Macromol. 2018, 113, 395–402. [Google Scholar] [CrossRef]
- Chan, S.-Y.; Choo, W.-S. Effect of extraction conditions on the yield and chemical properties of pectin from cocoa husks. Food Chem. 2013, 141, 3752–3758. [Google Scholar] [CrossRef]
- Pereira, P.H.F.; Oliveira, T.Í.S.; Rosa, M.F.; Cavalcante, F.L.; Moates, G.K.; Wellner, N.; Waldron, K.W.; Azeredo, H.M.C. Pectin extraction from pomegranate peels with citric acid. Int. J. Biol. Macromol. 2016, 88, 373–379. [Google Scholar] [CrossRef]
- Oliveira, T.Í.S.; Rosa, M.F.; Cavalcante, F.L.; Pereira, P.H.F.; Moates, G.K.; Wellner, N.; Mazzetto, S.E.; Waldron, K.W.; Azeredo, H.M.C. Optimization of pectin extraction from banana peels with citric acid by using response surface methodology. Food Chem. 2016, 198, 113–118. [Google Scholar] [CrossRef]
- Srivastava, P.; Malviya, R. Sources of pectin, extraction and its applications in pharmaceutical industry—An overview. Indian J. Nat. Prod. Resour. 2011, 2, 10–18. [Google Scholar]
- Gao, X.; Zhi, Y.; Sun, L.; Peng, X.; Zhang, T.; Xue, H.; Tai, G.; Zhou, Y. The inhibitory effects of a rhamnogalacturonan I (RG-I) domain from ginseng Pectin on galectin-3 and its structure-activity relationship. J. Biol. Chem. 2013, 288, 33953–33965. [Google Scholar] [CrossRef] [Green Version]
- Leclere, L.; Van Cutsem, P.; Michiels, C. Anti-cancer activities of pH- or heat-modified pectin. Front. Pharmacol. 2013, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leclere, L.; Fransolet, M.; Cote, F.; Cambier, P.; Arnould, T.; Van Cutsem, P.; Michiels, C. Heat-modified citrus pectin induces apoptosis-like cell death and autophagy in HepG2 and A549 cancer cells. PLoS ONE 2015, 10, e0115831. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Bailina, Y.; Ge, Z.; Ding, T.; Ye, X.; Liu, D. Effects of ultrasound and/or heating on the extraction of pectin from grapefruit peel. J. Food Eng. 2014, 126, 72–81. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Xu, Y.; Cao, Y.; Jiang, Z.; Ding, T.; Ye, X.; Liu, D. Ultrasound-assisted heating extraction of pectin from grapefruit peel: Optimization and comparison with the conventional method. Food Chem. 2015, 178, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, W.; Zou, M.; Lv, R.; Wang, D.; Hou, F.; Feng, H.; Ma, X.; Zhong, J.; Ding, T.; et al. Applications of power ultrasound in oriented modification and degradation of pectin: A review. J. Food Eng. 2018, 234, 98–107. [Google Scholar] [CrossRef]
- Zouambia, Y.; Ettoumi, K.Y.; Krea, M.; Moulai-Mostefa, N. A new approach for pectin extraction: Electromagnetic induction heating. Arab. J. Chem. 2017, 10, 480–487. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Cheng, H.; Zhi, Z.; Zhang, H.; Linhardt, R.J.; Zhang, F.; Chen, S.; Ye, X. Extraction temperature is a decisive factor for the properties of pectin. Food Hydrocoll. 2021, 112, 106160. [Google Scholar] [CrossRef]
- Blanchard, H.; Bum-Erdene, K.; Bohari, M.H.; Yu, X. Galectin-1 inhibitors and their potential therapeutic applications: A patent review. Expert Opin. Ther. Pat. 2016, 26, 537–554. [Google Scholar] [CrossRef] [PubMed]
- Laaf, D.; Bojarová, P.; Pelantová, H.; Křen, V.; Elling, L. Tailored Multivalent Neo-Glycoproteins: Synthesis, Evaluation, and Application of a Library of Galectin-3-Binding Glycan Ligands. Bioconjugate Chem. 2017, 28, 2832–2840. [Google Scholar] [CrossRef] [PubMed]
- Stegmayr, J.; Lepur, A.; Kahl-Knutson, B.; Aguilar-Moncayo, M.; Klyosov, A.A.; Field, R.A.; Oredsson, S.; Nilsson, U.J.; Leffler, H. Low or No Inhibitory Potency of the Canonical Galectin Carbohydrate-binding Site by Pectins and Galactomannans. J. Biol. Chem. 2016, 291, 13318–13334. [Google Scholar] [CrossRef] [Green Version]
- Gunning, A.P.; Bongaerts, R.J.M.; Morris, V.J. Recognition of galactan components of pectin by galectin-3. FASEB J. 2009, 23, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yu, L.; Shi, Y.; Lu, J.; Teng, H.; Zhou, Y.; Sun, L. Structural characterization of a rhamnogalacturonan I domain from ginseng and its inhibitory effect on galectin-3. Molecules 2017, 22, 1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Ma, P.; Shuai, M.; Huang, J.; Sun, C.; Yao, X.; Chen, Z.; Min, X.; Zhang, T. Analysis of the water-soluble polysaccharides from Camellia japonica pollen and their inhibitory effects on galectin-3 function. Int. J. Biol. Macromol. 2020, 159, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Wang, P.; Niu, B.; Kang, J. Environmental stress stability of pectin-stabilized resveratrol liposomes with different degree of esterification. Int. J. Biol. Macromol. 2018, 119, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, G.E.; Simas-Tosin, F.F.; Iacomini, M.; Gorin, P.A.J.; Cordeiro, L.M.C. Rheological behavior of high methoxyl pectin from the pulp of tamarillo fruit (Solanum betaceum). Carbohydr. Polym. 2016, 139, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, U.S.; Pietsch, V.L.; Rentschler, C.; Kurz, T.; Endreß, H.U.; Schuchmann, H.P. Influence of the degree of esterification on the emulsifying performance of conjugates formed between whey protein isolate and citrus pectin. Food Hydrocoll. 2016, 56, 1–8. [Google Scholar] [CrossRef]
- Schmidt, U.S.; Schütz, L.; Schuchmann, H.P. Interfacial and emulsifying properties of citrus pectin: Interaction of pH, ionic strength and degree of esterification. Food Hydrocoll. 2017, 62, 288–298. [Google Scholar] [CrossRef]
- Jacob, E.M.; Borah, A.; Jindal, A.; Pillai, S.C.; Yamamoto, Y.; Maekawa, T.; Kumar, D.N.S. Synthesis and characterization of citrus-derived pectin nanoparticles based on their degree of esterification. J. Mater. Res. 2020, 35, 1514–1522. [Google Scholar] [CrossRef]
- Wan, L.; Chen, Q.; Huang, M.; Liu, F.; Pan, S. Physiochemical, rheological and emulsifying properties of low methoxyl pectin prepared by high hydrostatic pressure-assisted enzymatic, conventional enzymatic, and alkaline de-esterification: A comparison study. Food Hydrocoll. 2019, 93, 146–155. [Google Scholar] [CrossRef]
- Dranca, F.; Oroian, M. Extraction, purification and characterization of pectin from alternative sources with potential technological applications. Food Res. Int. 2018, 113, 327–350. [Google Scholar] [CrossRef]
- Begum, R.; Yusof, Y.A.; Aziz, M.G.; Uddin, M.B. Structural and functional properties of pectin extracted from jackfruit (Artocarpus heterophyllus) waste: Effects of drying. Int. J. Food Prop. 2017, 20, S190–S201. [Google Scholar] [CrossRef] [Green Version]
- Karnik, D.; Wicker, L. Emulsion stability of sugar beet pectin fractions obtained by isopropanol fractionation. Food Hydrocoll. 2018, 74, 249–254. [Google Scholar] [CrossRef]
- Juttulapa, M.; Piriyaprasarth, S.; Takeuchi, H.; Sriamornsak, P. Effect of high-pressure homogenization on stability of emulsions containing zein and pectin. Asian J. Pharm. Sci. 2017, 12, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Petkowicz, C.L.O.; Vriesmann, L.C.; Williams, P.A. Pectins from food waste: Extraction, characterization and properties of watermelon rind pectin. Food Hydrocoll. 2017, 65, 57–67. [Google Scholar] [CrossRef]
- Picot-Allain, M.C.N.; Ramasawmy, B.; Emmambux, M.N. Extraction, Characterisation, and Application of Pectin from Tropical and Sub-Tropical Fruits: A Review. Food Rev. Int. 2020, 38, 282–312. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, X.; Ding, T.; Sun, X.; Xu, Y.; Liu, D. Ultrasound effects on the degradation kinetics, structure and rheological properties of apple pectin. Ultrason. Sonochemistry 2013, 20, 222–231. [Google Scholar] [CrossRef]
- Basanta, M.F.; Ponce, N.M.A.; Rojas, A.M.; Stortz, C.A. Effect of extraction time and temperature on the characteristics of loosely bound pectins from Japanese plum. Carbohydr. Polym. 2012, 89, 230–235. [Google Scholar] [CrossRef]
- Kosmala, M.; Milala, J.; Kołodziejczyk, K.; Markowski, J.; Zbrzeźniak, M.; Renard, C.M.G.C. Dietary fiber and cell wall polysaccharides from plum (Prunus domestica L.) fruit, juice and pomace: Comparison of composition and functional properties for three plum varieties. Food Res. Int. 2013, 54, 1787–1794. [Google Scholar] [CrossRef]
- Moreno, L.; Nascimento, R.F.; Zielinski, A.A.F.; Wosiacki, G.; Canteri, M.H.G. Extraction and characterization of pectic substances in Myrciaria cauliflora (Jaboticaba sabará) fruit. Rev. Strict. Sensu 2016, 1, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sayah, M.Y.; Chabir, R.; Benyahia, H.; Kandri, Y.R.; Chahdi, F.O.; Touzani, H.; Errachidi, F. Yield, esterification degree and molecular weight evaluation of pectins isolated from orange and grapefruit peels under different conditions. PLoS ONE 2016, 11, e0161751. [Google Scholar] [CrossRef] [Green Version]
- Hao, M.; Yuan, X.; Cheng, H.; Xue, H.; Zhang, T.; Zhou, Y.; Tai, G. Comparative studies on the anti-tumor activities of high temperature- and pH-modified citrus pectins. Food Funct. 2013, 4, 960–971. [Google Scholar] [CrossRef] [PubMed]
- Do Prado, S.B.R.; Melfi, P.R.; Castro-Alves, V.C.; Broetto, S.G.; Araújo, E.S.; Do Nascimento, J.R.O.; Fabi, J.P. Physiological degradation of pectin in papaya cell walls: Release of long chains galacturonans derived from insoluble fractions during postharvest fruit ripening. Front. Plant Sci. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prado, S.B.R.; Beukema, M.; Jermendi, E.; Schols, H.A.; de Vos, P.; Fabi, J.P. Pectin Interaction with Immune Receptors is Modulated by Ripening Process in Papayas. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Flores-Ibarra, A.; Vértesy, S.; Medrano, F.J.; Gabius, H.J.; Romero, A. Crystallization of a human galectin-3 variant with two ordered segments in the shortened N-terminal tail. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Zhang, T.; Wang, P.; Liu, F.; Tai, G.; Zhou, Y. The water network in galectin-3 ligand binding site guides inhibitor design. Acta Biochim. Biophys. Sin. 2015, 47, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Ippel, H.; Miller, M.C.; Vértesy, S.; Zheng, Y.; Cañada, F.J.; Suylen, D.; Umemoto, K.; Romanò, C.; Hackeng, T.; Tai, G.; et al. Intra- and intermolecular interactions of human galectin-3: Assessment by full-assignment-based NMR. Glycobiology 2016, 26, 888–903. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-J.; Chun, K.-H. Non-classical role of Galectin-3 in cancer progression: Translocation to nucleus by carbohydrate-recognition independent manner. BMB Rep. 2020, 53, 173–180. [Google Scholar] [CrossRef]
- Ruvolo, P.P. Galectin 3 as a guardian of the tumor microenvironment. Biochim. Biophys. Acta—Mol. Cell Res. 2016, 1863, 427–437. [Google Scholar] [CrossRef]
- Chan, Y.-C.; Lin, H.-Y.; Tu, Z.; Kuo, Y.-H.; Hsu, S.-T.D.; Lin, C.-H. Dissecting the structure–Activity relationship of galectin—Ligand interactions. Int. J. Mol. Sci. 2018, 19, 392. [Google Scholar] [CrossRef] [Green Version]
- Sehnal, D.; Bittrich, S.; Deshpande, M.; Svobodová, R.; Berka, K.; Bazgier, V.; Velankar, S.; Burley, S.K.; Koča, J.; Rose, A.S. Mol* Viewer: Modern web app for 3D visualization and analysis of large biomolecular structures. Nucleic Acids Res. 2021, 49, W431–W437. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Miller, M.C.; Zheng, Y.; Zhang, Z.; Xue, H.; Zhao, D.; Su, J.; Mayo, K.H.; Zhou, Y.; Tai, G. Macromolecular assemblies of complex polysaccharides with galectin-3 and their synergistic effects on function. Biochem. J. 2017, 474, 3849–3868. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.C.; Ippel, H.; Suylen, D.; Klyosov, A.A.; Traber, P.G.; Hackeng, T.; Mayo, K.H. Binding of polysaccharides to human galectin-3 at a noncanonical site in its carbohydrate recognition domain. Glycobiology 2015, 26, 88–99. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Kuwabara, R.; Beukema, M.; Ferrari, M.; de Haan, B.J.; Walvoort, M.T.C.; de Vos, P.; Smink, A.M. Low methyl-esterified pectin protects pancreatic β-cells against diabetes-induced oxidative and inflammatory stress via galectin-3. Carbohydr. Polym. 2020, 249, 116863. [Google Scholar] [CrossRef]
- Xu, G.-R.; Zhang, C.; Yang, H.-X.; Sun, J.-H.; Zhang, Y.; Yao, T.-T.; Li, Y.; Ruan, L.; An, R.; Li, A.-Y. Modified citrus pectin ameliorates myocardial fibrosis and inflammation via suppressing galectin-3 and TLR4/MyD88/NF-κB signaling pathway. Biomed. Pharmacother. 2020, 126, 110071. [Google Scholar] [CrossRef]
- Ilmer, M.; Mazurek, N.; Byrd, J.C.; Ramirez, K.; Hafley, M.; Alt, E.; Vykoukal, J.; Bresalier, R.S. Cell surface galectin-3 defines a subset of chemoresistant gastrointestinal tumor-initiating cancer cells with heightened stem cell characteristics. Cell Death Dis. 2016, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Zheng, Y.; Zhao, D.; Yan, J.; Sun, C.; Zhou, Y.; Tai, G. Multiple approaches to assess pectin binding to galectin-3. Int. J. Biol. Macromol. 2016, 91, 994–1001. [Google Scholar] [CrossRef]
- Zheng, Y.; Su, J.; Miller, M.C.; Geng, J.; Xu, X.; Zhang, T.; Mayzel, M.; Zhou, Y.; Mayo, K.H.; Tai, G. Topsy-turvy binding of negatively-charged homogalacturonan oligosaccharides to galectin-3. Glycobiology 2020, 31, 341–350. [Google Scholar] [CrossRef]
- Miller, M.C.; Zheng, Y.; Zhou, Y.; Tai, G.; Mayo, K.H. Galectin-3 binds selectively to the terminal, non-reducing end of β(1→4)-galactans, with overall affinity increasing with chain length. Glycobiology 2019, 29, 74–84. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, F.; Liu, X.; St. Ange, K.; Zhang, A.; Li, Q.; Linhardt, R.J. Isolation of a lectin binding rhamnogalacturonan-I containing pectic polysaccharide from pumpkin. Carbohydr. Polym. 2017, 163, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.C.; Zheng, Y.; Yan, J.; Zhou, Y.; Tai, G.; Mayo, K.H. Novel polysaccharide binding to the N-terminal tail of galectin-3 is likely modulated by proline isomerization. Glycobiology 2017, 27, 1038–1051. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, E.M.; Bovin, N.V. Specificity of human galectins on cell surfaces. Biochemistry 2015, 80, 846–856. [Google Scholar] [CrossRef] [PubMed]
- García Caballero, G.; Beckwith, D.; Shilova, N.V.; Gabba, A.; Kutzner, T.J.; Ludwig, A.-K.; Manning, J.C.; Kaltner, H.; Sinowatz, F.; Cudic, M.; et al. Influence of protein (human galectin-3) design on aspects of lectin activity. Histochem. Cell Biol. 2020, 154, 135–153. [Google Scholar] [CrossRef]
- Gao, X.; Zhi, Y.; Zhang, T.; Xue, H.; Wang, X.; Foday, A.D.; Tai, G.; Zhou, Y. Analysis of the neutral polysaccharide fraction of MCP and its inhibitory activity on galectin-3. Glycoconj. J. 2012, 29, 159–165. [Google Scholar] [CrossRef]
- Farhadi, S.A.; Liu, R.; Becker, M.W.; Phelps, E.A.; Hudalla, G.A. Physical tuning of galectin-3 signaling. Proc. Natl. Acad. Sci. USA 2021, 118, 1–10. [Google Scholar] [CrossRef]
- Cecioni, S.; Imberty, A.; Vidal, S. Glycomimetics versus multivalent glycoconjugates for the design of high affinity lectin ligands. Chem. Rev. 2015, 115, 525–561. [Google Scholar] [CrossRef] [PubMed]
- Hevey, R. Strategies for the development of glycomimetic drug candidates. Pharmaceuticals 2019, 12, 55. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, E.G.; Colquhoun, I.J.; Chau, H.K.; Hotchkiss, A.T.; Waldron, K.W.; Morris, V.J.; Belshaw, N.J. Modified sugar beet pectin induces apoptosis of colon cancer cells via an interaction with the neutral sugar side-chains. Carbohydr. Polym. 2016, 136, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Li, S.; Fan, Y.; Gao, X.; Hao, M.; Wang, J.; Zhang, X.; Tai, G.; Zhou, Y. Comparative studies of the antiproliferative effects of ginseng polysaccharides on HT-29 human colon cancer cells. Med. Oncol. 2011, 28, 175–181. [Google Scholar] [CrossRef]
- Pynam, H.; Dharmesh, S.M. A xylorhamnoarabinogalactan I from Bael (Aegle marmelos L.) modulates UV/DMBA induced skin cancer via galectin-3 & gut microbiota. J. Funct. Foods 2019, 60, 103425. [Google Scholar] [CrossRef]
- Popov, S.V.; Ovodov, Y.S. Polypotency of the immunomodulatory effect of pectins. Biochemistry 2013, 78, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Liu, D.-D.; Ning, H.-M.; Liu, D.; Sun, J.-Y.; Huang, X.-J.; Dong, Y.; Geng, M.-Y.; Yun, S.-F.; Yan, J.; et al. Modified citrus pectin inhibited bladder tumor growth through downregulation of galectin-3. Acta Pharmacol. Sin. 2018, 39, 1885–1893. [Google Scholar] [CrossRef] [Green Version]
- Hossein, G.; Halvaei, S.; Heidarian, Y.; Dehghani-Ghobadi, Z.; Hassani, M.; Hosseini, H.; Naderi, N.; Sheikh Hassani, S. Pectasol-C Modified Citrus Pectin targets Galectin-3-induced STAT3 activation and synergize paclitaxel cytotoxic effect on ovarian cancer spheroids. Cancer Med. 2019, 8, 4315–4329. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elsaad, N.M.; Elkashef, W.F. Modified citrus pectin stops progression of liver fibrosis by inhibiting galectin-3 and inducing apoptosis of stellate cells. Can. J. Physiol. Pharmacol. 2016, 94, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martinez, E.; Ibarrola, J.; Calvier, L.; Fernandez-Celis, A.; Leroy, C.; Cachofeiro, V.; Rossignol, P.; Lopez-Andres, N. Galectin-3 blockade reduces renal fibrosis in two normotensive experimental models of renal damage. PLoS ONE 2016, 11, e0166272. [Google Scholar] [CrossRef]
- Calvier, L.; Miana, M.; Reboul, P.; Cachofeiro, V.; Martinez-Martinez, E.; De Boer, R.A.; Poirier, F.; Lacolley, P.; Zannad, F.; Rossignol, P.; et al. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-H.; Chou, C.-H.; Wu, X.-M.; Chang, Y.-Y.; Hung, C.-S.; Chen, Y.-H.; Tzeng, Y.-L.; Wu, V.-C.; Ho, Y.-L.; Hsieh, F.-J.; et al. Aldosterone induced galectin-3 secretion in vitro and in vivo: From cells to humans. PLoS ONE 2014, 9, e95254. [Google Scholar] [CrossRef] [PubMed]
- Calvier, L.; Martinez-Martinez, E.; Miana, M.; Cachofeiro, V.; Rousseau, E.; Sádaba, J.R.; Zannad, F.; Rossignol, P.; López-Andrés, N. The impact of galectin-3 inhibition on aldosterone-induced cardiac and renal injuries. JACC Heart Fail. 2015, 3, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Yang, S.; Li, J.-C.; Feng, J.-X. Galectin 3 inhibition attenuates renal injury progression in cisplatin-induced nephrotoxicity. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prud’homme, M.; Coutrot, M.; Michel, T.; Boutin, L.; Genest, M.; Poirier, F.; Launay, J.M.; Kane, B.; Kinugasa, S.; Prakoura, N.; et al. Acute Kidney Injury Induces Remote Cardiac Damage and Dysfunction through the Galectin-3 Pathway. JACC Basic Transl. Sci. 2019, 4, 717–732. [Google Scholar] [CrossRef]
- Ibarrola, J.; Matilla, L.; Martínez-Martínez, E.; Gueret, A.; Fernández-Celis, A.; Henry, J.P.; Nicol, L.; Jaisser, F.; Mulder, P.; Ouvrard-Pascaud, A.; et al. Myocardial Injury after Ischemia/Reperfusion Is Attenuated by Pharmacological Galectin-3 Inhibition. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Li, S.; Hao, X.; Zhang, Y.; Deng, W. Perindopril and a Galectin-3 inhibitor improve ischemic heart failure in rabbits by reducing gal-3 expression and myocardial fibrosis. Front. Physiol. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vergaro, G.; Prud’Homme, M.; Fazal, L.; Merval, R.; Passino, C.; Emdin, M.; Samuel, J.L.; Cohen Solal, A.; Delcayre, C. Inhibition of Galectin-3 Pathway Prevents Isoproterenol-Induced Left Ventricular Dysfunction and Fibrosis in Mice. Hypertension 2016, 67, 606–612. [Google Scholar] [CrossRef] [Green Version]
- Ibarrola, J.; Martínez-Martínez, E.; Sádaba, J.R.; Arrieta, V.; García-Peña, A.; Álvarez, V.; Fernández-Celis, A.; Gainza, A.; Rossignol, P.; Ramos, V.C.; et al. Beneficial effects of galectin-3 blockade in vascular and aortic valve alterations in an experimental pressure overload model. Int. J. Mol. Sci. 2017, 18, 1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, H.; Zhao, Z.; Lin, Z.; Geng, J.; Guan, Y.; Song, C.; Zhou, Y.; Tai, G. Selective effects of ginseng pectins on galectin-3-mediated T cell activation and apoptosis. Carbohydr. Polym. 2019, 219, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.S.; Liu, E.; Paniagua, S.M.; Sarma, A.A.; Zampierollo, G.; López, B.; Díez, J.; Wang, T.J.; Ho, J.E. Galectin-3 Inhibition With Modified Citrus Pectin in Hypertension. JACC Basic Transl. Sci. 2021, 6, 12–21. [Google Scholar] [CrossRef]
- Portacci, A.; Diaferia, F.; Santomasi, C.; Dragonieri, S.; Boniello, E.; Di Serio, F.; Carpagnano, G.E. Galectin-3 as prognostic biomarker in patients with COVID-19 acute respiratory failure. Respir. Med. 2021, 187, 106556. [Google Scholar] [CrossRef]
- Kuśnierz-Cabala, B.; Maziarz, B.; Dumnicka, P.; Dembiński, M.; Kapusta, M.; Bociąga-Jasik, M.; Winiarski, M.; Garlicki, A.; Grodzicki, T.; Kukla, M. Diagnostic significance of serum galectin-3 in hospitalized patients with COVID-19—A preliminary study. Biomolecules 2021, 11, 1136. [Google Scholar] [CrossRef] [PubMed]
- Gaughan, A.E.; Sethi, T.; Quinn, T.; Hirani, N.; Mills, A.; Annya, M.; Mackinnon, A.; Aslanis, V.; Li, F.; Connor, R.O.; et al. GB0139, an inhaled small molecule inhibitor of galectin-3, in COVID-19 pneumonitis: A randomised, controlled, open-label, phase 2a experimental medicine trial of safety, pharmacokinetics, and potential therapeutic value. medRxiv 2022. [Google Scholar] [CrossRef]
- Sakurai, M.H.; Matsumoto, T.; Kiyohara, H.; Yamada, H. Detection and tissue distribution of anti-ulcer peptic polysaccharides from Bepleurum falcatum by polyclonal antibody. Planta Med. 1996, 62, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Busato, B.; de Almeida Abreu, E.C.; de Oliveira Petkowicz, C.L.; Martinez, G.R.; Noleto, G.R. Pectin from Brassica oleracea var. italica triggers immunomodulating effects in vivo. Int. J. Biol. Macromol. 2020, 161, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, P.; Zhang, H. Pectin in cancer therapy: A review. Trends Food Sci. Technol. 2015, 44, 258–271. [Google Scholar] [CrossRef]
- Majee, S.B.; Avlani, D.; Ghosh, P.; Biswas, G.R. Therapeutic and pharmaceutical benefits of native and modified plant pectin. J. Med. Plants Res. 2018, 12, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.-Y.; Huang, Z.-L.; Yang, G.-H.; Lu, W.-Q.; Yu, N.-R. Inhibitory effect of modified citrus pectin on liver metastases in a mouse colon cancer model. World J. Gastroenterol. 2008, 14, 7386–7391. [Google Scholar] [CrossRef]
- Courts, F.L. Profiling of modified citrus pectin oligosaccharide transport across Caco-2 cell monolayers. PharmaNutrition 2013, 1, 22–31. [Google Scholar] [CrossRef]
- Huang, P.-H.; Fu, L.-C.; Huang, C.-S.; Wang, Y.-T.; Wu, M.-C. The uptake of oligogalacturonide and its effect on growth inhibition, lactate dehydrogenase activity and galactin-3 release of human cancer cells. Food Chem. 2012, 132, 1987–1995. [Google Scholar] [CrossRef]
- Mabbott, N.A.; Donaldson, D.S.; Ohno, H.; Williams, I.R.; Mahajan, A. Microfold (M) cells: Important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013, 6, 666–677. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Sun, L.; Yang, S.; He, C.; Tai, G.; Zhou, Y. The roles and mechanisms of homogalacturonan and rhamnogalacturonan I pectins on the inhibition of cell migration. Int. J. Biol. Macromol. 2018, 106, 207–217. [Google Scholar] [CrossRef]
- Shao, A.; Wu, H.; Zhang, J. Letter by Shao et al Regarding Article, “Modified Citrus Pectin Prevents Blood-Brain Barrier Disruption in Mouse Subarachnoid Hemorrhage by Inhibiting Galectin-3”. J. Biol. Chem. 2019, 50, e22. [Google Scholar] [CrossRef]
- Leffler, H. Letter by Leffler Regarding Article, “Modified Citrus Pectin Prevents Blood-Brain Barrier Disruption in Mouse Subarachnoid Hemorrhage by Inhibiting Galectin-3”. J. Biol. Chem. 2019, 50, e136. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Liu, L.; Nakano, F.; Kawakita, F.; Kanamaru, H.; Nakatsuka, Y.; Okada, T.; Suzuki, H. Modified citrus pectin prevents blood-brain barrier disruption in mouse Subarachnoid hemorrhage by inhibiting Galectin-3. Stroke 2018, 49, 2743–2751. [Google Scholar] [CrossRef]
- Hirani, N.; MacKinnon, A.C.; Nicol, L.; Ford, P.; Schambye, H.; Pedersen, A.; Nilsson, U.J.; Leffler, H.; Sethi, T.; Tantawi, S.; et al. Target inhibition of galectin-3 by inhaled TD139 in patients with idiopathic pulmonary fibrosis. Eur. Respir. J. 2021, 57, 1–13. [Google Scholar] [CrossRef]
- Bumba, L.; Laaf, D.; Spiwok, V.; Elling, L.; Křen, V.; Bojarová, P. Poly-N-acetyllactosamine Neo-glycoproteins as nanomolar ligands of human galectin-3: Binding kinetics and modeling. Int. J. Mol. Sci. 2018, 19, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laaf, D.; Steffens, H.; Pelantová, H.; Bojarová, P.; Křen, V.; Elling, L. Chemo-Enzymatic Synthesis of Branched N-Acetyllactosamine Glycan Oligomers for Galectin-3 Inhibition. Adv. Synth. Catal. 2017, 359, 4015–4024. [Google Scholar] [CrossRef]
- Fischöder, T.; Laaf, D.; Dey, C.; Elling, L. Enzymatic synthesis of N-acetyllactosamine (LacNAc) type 1 oligomers and characterization as multivalent galectin ligands. Molecules 2017, 22, 1320. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, D.; Barak, S. Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: A review. Int. J. Biol. Macromol. 2013, 61, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Havenaar, R. Intestinal health functions of colonic microbial metabolites: A review. Benef. Microbes 2011, 2, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; De los Reyes-Gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivaprakasam, S.; Gurav, A.; Paschall, A.V.; Coe, G.L.; Chaudhary, K.; Cai, Y.; Kolhe, R.; Martin, P.; Browning, D.; Huang, L.; et al. An essential role of Ffar2 (Gpr43) in dietary fibre-mediated promotion of healthy composition of gut microbiota and suppression of intestinal carcinogenesis. Oncogenesis 2016, 5, e238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivaprakasam, S.; Prasad, P.D.; Singh, N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol. Ther. 2016, 164, 144–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 2013, 145, 396–406. [Google Scholar] [CrossRef]
- Beukema, M.; Faas, M.M.; de Vos, P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: Impact via gut microbiota and direct effects on immune cells. Exp. Mol. Med. 2020, 52, 1364–1376. [Google Scholar] [CrossRef]
- Tian, L.; Scholte, J.; Borewicz, K.; van den Bogert, B.; Smidt, H.; Scheurink, A.J.W.; Gruppen, H.; Schols, H.A. Effects of pectin supplementation on the fermentation patterns of different structural carbohydrates in rats. Mol. Nutr. Food Res. 2016, 60, 2256–2266. [Google Scholar] [CrossRef]
- Tian, L.; Bruggeman, G.; van den Berg, M.; Borewicz, K.; Scheurink, A.J.W.; Bruininx, E.; de Vos, P.; Smidt, H.; Schols, H.A.; Gruppen, H. Effects of pectin on fermentation characteristics, carbohydrate utilization, and microbial community composition in the gastrointestinal tract of weaning pigs. Mol. Nutr. Food Res. 2017, 61, 1–10. [Google Scholar] [CrossRef]
- Ferreira-Lazarte, A.; Moreno, F.J.; Cueva, C.; Gil-Sánchez, I.; Villamiel, M. Behaviour of citrus pectin during its gastrointestinal digestion and fermentation in a dynamic simulator (simgi®). Carbohydr. Polym. 2019, 207, 382–390. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Liang, R.-H.; Liu, W.; Li, T.; Liu, C.-M.; Wu, S.-S.; Wang, Z.-J. Pectic-oligosaccharides prepared by dynamic high-pressure microfluidization and their in vitro fermentation properties. Carbohydr. Polym. 2013, 91, 175–182. [Google Scholar] [CrossRef]
- Onumpai, C.; Kolida, S.; Bonnin, E.; Rastall, R.A. Microbial utilization and selectivity of pectin fractions with various structures. Appl. Environ. Microbiol. 2011, 77, 5747–5754. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Siles, M.; Khan, T.M.; Duncan, S.H.; Harmsen, H.J.M.; Garcia-Gil, L.J.; Flint, H.J. Cultured representatives of two major phylogroups of human colonic Faecalibacterium prausnitzii can utilize pectin, uronic acids, and host-derived substrates for growth. Appl. Environ. Microbiol. 2012, 78, 420–428. [Google Scholar] [CrossRef] [Green Version]
- Scott, K.P.; Martin, J.C.; Duncan, S.H.; Flint, H.J. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol. Ecol. 2014, 87, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Merheb, R.; Abdel-Massih, R.M.; Karam, M.C. Immunomodulatory effect of natural and modified Citrus pectin on cytokine levels in the spleen of BALB/c mice. Int. J. Biol. Macromol. 2019, 121, 1–5. [Google Scholar] [CrossRef]
- Amorim, J.C.; Vriesmann, L.C.; Petkowicz, C.L.O.; Martinez, G.R.; Noleto, G.R. Modified pectin from Theobroma cacao induces potent pro-inflammatory activity in murine peritoneal macrophage. Int. J. Biol. Macromol. 2016, 92, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, G.E.; Winnischofer, S.M.B.; Ramirez, M.I.; Iacomini, M.; Cordeiro, L.M.C. The influence of sweet pepper pectin structural characteristics on cytokine secretion by THP-1 macrophages. Food Res. Int. 2017, 102, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Popov, S.V.; Ovodova, R.G.; Golovchenko, V.V.; Popova, G.Y.; Viatyasev, F.V.; Shashkov, A.S.; Ovodov, Y.S. Chemical composition and anti-inflammatory activity of a pectic polysaccharide isolated from sweet pepper using a simulated gastric medium. Food Chem. 2011, 124, 309–315. [Google Scholar] [CrossRef]
- Ishisono, K.; Yabe, T.; Kitaguchi, K. Citrus pectin attenuates endotoxin shock via suppression of Toll-like receptor signaling in Peyer’s patch myeloid cells. J. Nutr. Biochem. 2017, 50, 38–45. [Google Scholar] [CrossRef]
- Vogt, L.M.; Sahasrabudhe, N.M.; Ramasamy, U.; Meyer, D.; Pullens, G.; Faas, M.M.; Venema, K.; Schols, H.A.; de Vos, P. The impact of lemon pectin characteristics on TLR activation and T84 intestinal epithelial cell barrier function. J. Funct. Foods 2016, 22, 398–407. [Google Scholar] [CrossRef]
- Wang, H.; Bi, H.; Gao, T.; Zhao, B.; Ni, W.; Liu, J. A homogalacturonan from Hippophae rhamnoides L. Berries enhance immunomodulatory activity through TLR4/MyD88 pathway mediated activation of macrophages. Int. J. Biol. Macromol. 2018, 107, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Park, S.N.; Noh, K.T.; Jeong, Y.-I.; Jung, I.D.; Kang, H.K.; Cha, G.S.; Lee, S.J.; Seo, J.K.; Kang, D.H.; Hwang, T.-H.; et al. Rhamnogalacturonan II is a Toll-like receptor 4 agonist that inhibits tumor growth by activating dendritic cell-mediated CD8+ T cells. Exp. Mol. Med. 2013, 45, e8. [Google Scholar] [CrossRef] [PubMed]
- Sahasrabudhe, N.M.; Beukema, M.; Tian, L.; Troost, B.; Scholte, J.; Bruininx, E.; Bruggeman, G.; van den Berg, M.; Scheurink, A.; Schols, H.A.; et al. Dietary fiber pectin directly blocks toll-like receptor 2—1 and prevents doxorubicin-induced ileitis. Front. Immunol. 2018, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Kuwabara, R.; Chica, C.E.N.; Smink, A.M.; Koster, T.; Medina, J.D.; de Haan, B.J.; Beukema, M.; Lakey, J.R.T.; García, A.J.; et al. Toll-like receptor 2-modulating pectin-polymers in alginate-based microcapsules attenuate immune responses and support islet-xenograft survival. Biomaterials 2021, 266, 120460. [Google Scholar] [CrossRef] [PubMed]
- Kolatsi-Joannou, M.; Price, K.L.; Winyard, P.J.; Long, D.A. Modified citrus pectin reduces galectin-3 expression and disease severity in experimental acute kidney injury. PLoS ONE 2011, 6, e18683. [Google Scholar] [CrossRef] [Green Version]
- Mai, C.W.; Kang, Y.B.; Pichika, M.R. Should a Toll-like receptor 4 (TLR-4) agonist or antagonist be designed to treat cancer? TLR-4: Its expression and effects in the ten most common cancers. OncoTargets Ther. 2013, 6, 1573–1587. [Google Scholar] [CrossRef] [Green Version]
- Kaczanowska, S.; Joseph, A.M.; Davila, E. TLR agonists: Our best frenemy in cancer immunotherapy. J. Leukoc. Biol. 2013, 93, 847–863. [Google Scholar] [CrossRef] [Green Version]
- Pradere, J.P.; Dapito, D.H.; Schwabe, R.F. The Yin and Yang of Toll-like receptors in cancer. Oncogene 2014, 33, 3485–3495. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Gao, T.; Du, Y.; Yang, H.; Wei, L.; Bi, H.; Ni, W. Anticancer and immunostimulating activities of a novel homogalacturonan from Hippophae rhamnoides L. berry. Carbohydr. Polym. 2015, 131, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Sato-Kaneko, F.; Yao, S.; Ahmadi, A.; Zhang, S.S.; Hosoya, T.; Kaneda, M.M.; Varner, J.A.; Pu, M.; Messer, K.S.; Guiducci, C.; et al. Combination immunotherapy with TLR agonists and checkpoint inhibitors suppresses head and neck cancer. JCI Insight 2017, 2, 1–18. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Karim, N.; Gowd, V.; Xie, J.; Zheng, X.; Chen, W. Pectin-chitosan conjugated nanoliposome as a promising delivery system for neohesperidin: Characterization, release behavior, cellular uptake, and antioxidant property. Food Hydrocoll. 2019, 95, 432–444. [Google Scholar] [CrossRef]






| Authors | Polysaccharide Residue | Analysis Method | Binding Evaluation |
|---|---|---|---|
| Wu et al., 2020 [33] | RG-I from citrus canning process water | Surface plasmon resonance | Smooth binding curve through SPR with decreased affinity with galactan side-chain removal |
| Zhang et al., 2016 [34] | MCP, RG-I-4, and p-galactan | Gal-3 hemagglutination, bio-layer interferometry, and surface plasmon resonance | RG-I-4 demonstrated higher Gal-3 avidity in comparison to the other two polysaccharides, with a KD at sub-micromolar range (RG-I-4 and p-galactan), but no significant result when testing competitive assays with known S-face inhibitors such as lactose |
| Gao et al., 2013 [40] | Ginseng RG-I-4 domain | Gal-3 hemagglutination and surface plasmon resonance | RG-I-4 inhibited G3H and was bound specifically to CRD with high affinity with Ara residue location in the RG-I, changing the activity detected at the G3H assay |
| Gunning, Bongaerts, Morris et al., 2009 [51] | RG-I, PG, and galactans | Atomic force microscopy, fluorescence microscopy, nuclear magnetic resonance, and flow cytometry | Galactan binding to Gal-3 is lectin-saccharide highly specific, while RG-I has low specificity, and PG was not specific. The data suggest that the lesser “sterical crowding” of the galactans alongside its beta-1,4 linear chain could be the reason for the better performance observed |
| Shi et al., 2017 [52] | Ginseng RG-I-3A domain | Bio-layer interferometry, Gal-3 hemagglutination | Binding kinetics of RG-I-3A showed a high binding affinity with a KD of 28 nM through and also presented notable G3H inhibition |
| Zhang et al., 2017 [83] | MCP-derived RG-I and HG portions | Gal-3 hemagglutination, bio-layer interferometry, ELISA, and nuclear magnetic resonance | Gal-3 bound to both portions separately but with a much more notable avidity when a combination of them (RG + HG) is performed, suggesting that this interaction exposes more binding sites at the lectin |
| Miller et al., 2015 [84] | Galactomannans (GM) and polymannan | Nuclear magnetic resonance | The primary binding surface of the GM’s located mainly at F-face beta-sheets (7,8 and 9) |
| Zheng et al., 2020 [89] | MCP-derived HGs of varying molecular weights | Nuclear magnetic resonance heteronuclear single quantum coherence spectroscopy and crystallography | Higher molecular weight HGs demonstrated more perturbances at F-face resonances and involved more S-face beta-sheets at the binding footprint. A possible binding of Gal-3 to the non-terminal HG sites is suggested, and it is shown a different S-face binding pattern of HG’s compared to lactose |
| Miller et al., 2019 [90] | Galactan oligosaccharides of varying chain lengths | Nuclear magnetic resonance heteronuclear single quantum coherence spectroscopy | Binding affinity at the terminal non-reducing end of the galactans in the CRD S-face (beta-sheets 4, 5, and 6 chemical shifts mostly) increases with the increase in chain length |
| Zhao et al., 2017 [91] | Pumpkin RG-I-containing pectin | Surface plasmon resonance | Moderate binding affinity towards Gal-3 through SPR, with a fast association between protein and polysaccharide (KA) and slow dissociation (KD) |
| Miller et al., 2017 [92] | Ginseng RG-I-4 domain | Nuclear magnetic resonance heteronuclear single quantum coherence spectroscopy | Epitopes from RG-I-4 bind to three different labeled Gal-3 sites, two at the CRD and another one at NT. At lower concentrations, the F-face site is more activated, turning to S-face at higher ones |
| Authors | Treatment | Study Type | Treatment Target | Observed Experimental Effects |
|---|---|---|---|---|
| Pedrosa, Lopes and Fabi, 2020 [7] | Papaya pectin acid and neutral fractions | In vitro | HCT 116, HT-29, and HCT-116 Gal-3−/− | Gal-3-mediated agglutination inhibition, cell viability decrease in both WT and knockout cells (suggesting Gal-3 independent pathways) |
| Chen et al., 2018 [10] | SCFAs | In vivo | Male apoE−/− mice | Stimulation of Lxrα mediated genes expression related to intestinal cholesterol uptake and excretion; improved blood lipid profiles and anti-atherosclerotic property |
| Li, Zhang, and Yang 2018 [11] | CP | In vivo | Healthy male C57BL/6J mice | Pectin-supplemented high-fat diet mice had reduced lower liver damage, lipid accumulation, and total serum triglyceride |
| Brouns et al., 2012 [12] | Different DM and MW apple and citrus pectin (CP) | Human intervention | Mildly hyper-cholesterolemic men and women | Higher DM apple and citrus pectin lowered between 7 and 10% low-density lipoprotein cholesterol (LDL-C) compared to control |
| Liu et al., 2016 [13] | CP | In vivo | Male Sprague-Dawley rats with induced type 2 diabetes | Enhanced glucose tolerance, blood lipid levels, reduced insulin resistance, pAKT expression upregulation, and glycogen synthase kinase 3 β (GSK3β) downregulation |
| Fotschki et al., 2014 [14] | Apple fiber (low pectin) | In vivo | Male Wistar rats | Disaccharidase activity reduction, higher SCFA production, reduced serum glucose concentration |
| Prado et al., 2019 [32] | Chelate-soluble fraction of papaya pectin | In vitro | HCT 116 and HT-29 human colon cancer cells | Gal-3-mediated agglutination inhibition, similar to lactose control; pre-treatment with lactose suggests cell Gal-3 independent proliferation reduction for one of the fractions (3CSF) |
| Wu et al., 2020 [33] | CP fragments | In vitro | MCF-7 human breast cancer and A549 human lung carcinoma | Significant binding affinities to Gal-3; dose-responsive cell proliferation inhibition in vitro, not necessarily related to Gal-3 |
| Gao et al., 2013 [40] | MCP, ginseng pectin fractions, potato galactans, and RG-I | In vitro | HT-29 human colon cancer cell line | RG I-4 from ginseng strongly inhibited Gal-3 mediated hemagglutination; better inhibition of cell adhesion and homotypic cell aggregation than lactose |
| Stegmayr et al., 2016 [50] | MCP | In vitro | JIMT-1 breast cancer cells | No Gal-3 inhibition was detected; however, MCP pre-incubation resulted in the accumulation of Gal-3 molecules around intracellular vesicles |
| Prado et al., 2020 [73] | Papaya pectins from different ripening periods | In vitro | THP-1 human monocytic cell | Different TLR’s activation and inhibition depend on the ripening period |
| Hu et al., 2020 [85] | Lemon pectin | In vitro | Human pancreatic beta-cell | Unspecific and unspecified reduction of deleterious effects of inflammatory cytokines with very low (5%) degree of esterification pectin at cell culture |
| Xu et al., 2020 [86] | MCP | In vivo | Male Wistar rats | Down-regulation of Gal-3, TLR, and MyD88, decreased expression of IL-1β, IL-18, and TNF-α |
| Maxwell et al., 2016 [99] | Sugar beet and CP | In vitro | HT-29 human colon cancer cell line | Cell proliferation control and induction of apoptosis |
| Pynam and Dharmesh, 2019 [101] | Bael fruit pectin fragments | In vitro and in vivo | Healthy Swiss albino mice and B16F10 cell line | Microbiota protection, tyrosinase down-regulation, Gal-3 binding, downregulation of Gal-3 gene, IL10 and IL17 cytokines |
| Fang et al., 2018 [103] | MCP | In vitro | Human urinary bladder cancer (UBC) cells | Gal-3 down-regulation and inactivation of Akt signaling pathway, a decrease in Cyclin B1, G2/M phase arrest, Caspase-3 activation |
| Hossein et al., 2019 [104] | MCP | In vitro | SKOV-3 and SOC (serous ovarian cancer) cells | Synergistic effect of PTX and MCP increasing caspase-3 activity and decreasing cyclin D1 expression level |
| Abu-Elsaad and Elkashef, 2016 [105] | MCP | In vivo | Adult male Sprague-Dawley rats | Decreased liver fibrosis and necroinflammation, a decrease in MDA, TIMP-1, Col1A1, and Gal-3, increase in Caspase-3, gluthatione, and superoxide dismutase expression |
| Martinez-Martinez et al., 2016 [106] | MCP | In vivo | Adult male Wistar rats | Attenuation of renal fibrosis-related biomarkers, osteopontin, cytokine A2, albuminuria and TGF-β1 |
| Calvier et al., 2015 [109] | MCP | In vivo | Adult male Wistar rats, C57BJ6 WT and Gal-3−/− mice | Reverted fibrosing markers and Gal-3 augmentation levels, similarly to spironolactone |
| Li et al., 2018 [110] | MCP | In vitro and in vivo | HEK293 cells and C57BL/6 male mice | Amelioration of renal interstitial fibrosis, lower collagen I and fibronectin in the kidney, reduced IL-1β mRNA levels, lower Gal-3 expression |
| Prud’homme et al., 2019 [111] | MCP | Cohort and in vivo | C57BL6/J and C57BL6/J Gal-3 KO male mice | Cardiac fibrosis induced by model prevented by MCP treatment, IL-1β level maintained, protected, treated mice against renal inflammation |
| Ibarrola et al., 2019 [112] | MCP | In vivo | Male Wistar rats | BNP serum level normalization, lower Gal-3 cardiac expression, reticulocalbin-3 and fumarase in the myocardium, IL-1β and CRP in serum |
| Li et al., 2019 [113] | MCP and perindopril | In vivo | New Zealand male rabbits | Gal-3, collagen I, and III downregulation |
| Vergaro et al., 2016 [114] | MCP | In vivo | Transgenic mice with aldosterone synthase gene overexpression | Reduced cardiac hypertrophy, fibrosis, Coll-1, and Coll-3 genes expression and also enhanced anti-inflammatory and anti-fibrotic effects when synergistically acting with Canrenoate |
| Ibarrola et al., 2017 [115] | MCP | In vivo | Male Wistar rats | Gal-3, mRNA expression normalized, collagen I, fibronectin, α-SMA, TGF-β1, and CTGF mRNA expression reduced compared to pressure overload group, vascular inflammatory markers expression was also controlled |
| Xue et al., 2019 [116] | Ginseng pectin fractions | In vitro and In vivo | Jurkat (human leukemia cells) and male IRC mice | MCP inhibited IL-2 expression, and the three pectin fractions utilized reversed cleaved caspase-3 formation alongside lactose. MCP and ginseng pectins inhibited ROS production in vitro. Reduced tumor weight and increased IL-2 secretion in vivo |
| Lau et al., 2021 [117] | MCP | Interventional trial | Participants with high Gal-3 levels and hypertension | MCP had no impact regarding attenuating of cardiac-related risk factors |
| Busato et al., 2020 [122] | Broccoli stalks pectin | In vitro and in vivo | Female albino swiss mice and peritoneal exsudate cells | Macrophage activation and higher phagocytic activity; IL-10 presence was higher at peritoneal fluid in vivo, but not at in vitro model |
| Liu et al., 2008 [125] | MCP | In vitro and in vivo | CT-26 cells and Balb/c female mice | MCP did not alter Gal-3 expression at metastatic liver cells, although it did inhibit tumor growth and metastatic rate |
| Courts, 2013 [126] | MCP | In vitro | Caco-2 monolayer | MCP fragments were absorbed through paracellular and to a lower degree by transcellular transports at in vitro culture |
| Huang et al., 2012 [127] | Enzyme-treated CP | In vitro and In vivo | HepG2, A549, Colo 205, and HEK293 cells, BALB/c mice | Altered membrane permeability (LDH release) in the cancer cell lines; low weight oligogalacturonide was absorbed by the mice and the tumor cells, enhancing Gal-3 release to the medium |
| Fan et al., 2018 [129] | Ginseng RG-I enriched pectins | In vitro | L-929 fibroblast cells | Modulation of cell migration and adhesion, independent of Gal-3 |
| Nishikawa et al., 2018 [130] | Modified citrus pectin (MCP) | In vivo | Male C57BL/6 mice | Attenuated blood-brain barrier disruption Gal-3 upregulation, inactivation of ERK 1/2, STAT and MMP |
| Sivaprakasam et al., 2016 [143] | 2% inulin, 2% pectin, and 1% cellulose | In vivo | Human colon cancer tissue and Ffar-2−/− C57BL/6J mice | Microbiota modulation, promotion of Bifidobacterium growth, and reduction of Prevotellaceae |
| Kim et al., 2013 [144] | SCFAs | In vivo | WT, GPR41−/− and GPR43−/− mice | Activation of intestinal epithelial cells to produce chemokines and cytokines, GPR’s were essential in T effector cell activation and signaling pathways |
| Tian et al., 2016 [146] | Sugar beet, soy, low DM, and high DM citrus pectin | In vivo | Male Wistar rats | More stimulation of Lactobacillus and Lachnospiraceae growth in sugar beet pectin, higher production of SCFA’s for low DM citrus and soy pectin |
| Tian et al., 2017 [147] | Low DM and high DM citrus pectin | In vivo | Piglets | The slower fermentation process, alteration of main fermentation region, and higher Bacteroidetes predominance |
| Ferreira-Lazarte et al., 2019 [148] | CP | In vitro | Dynamic gastric simulator with healthy volunteer fecal slurry donated | Growth stimulation of Bifidobacterium spp., Bacteroides spp., and Faecalobacterium prausnitzii, high increase in acetate and butyrate production |
| Chen et al., 2013 [149] | Apple pectin oligosaccharides | In vitro | Fecal batch culture fermentation | Increased numbers of Lactobacillus and Bifidobacteria, a higher concentration of acetic, lactic, and propionic acid decreased number of Clostridia and Bacteroides |
| Onumpai et al., 2011 [150] | Potato galactan, methylated citrus pectin, beet arabinan, Arabidopsis thaliana RG-I | In vitro | Fecal batch culture fermentation | Higher Bifidobacterium populations and higher SCFA’s yield increased Bacteroides-Prevotella groups |
| Merheb, Abdel-Massih, and Karam, 2019 [153] | CP and MCP | In vivo | Female BALB/c mice | Upregulation of IL-17, IFN-γ, and TNF-α through IL-4 cytokine secretion in the spleen |
| Amorim et al., 2016 [154] | Theobroma cacao pod husk modified pectin | In vivo | Female albino Swiss mice | Promotion of macrophage differentiation, nitric oxide production, and upregulation of IL-12, TNF-α, and IL-10 secretion |
| Do Nascimento et al., 2017 [155] | Sweet pepper pectin | In vitro | THP-1 human monocytic cell | Modulation of TNF-α, IL-1β, and IL-10 production and secretion |
| Popov et al., 2011 [156] | Sweet pepper pectin | In vivo | Male BALB/c mice | Higher IL-10 production with lower TNF-α release |
| Ishisono et al., 2017 [157] | CP | In vivo | Male C57BL/6 mice | Suppression of IL-6 secretion from TLR activated macrophages and CD11c+ cells |
| Vogt et al., 2016 [158] | Different DM lemon pectin | In vitro | T84 intestinal epithelial cells | NF-kB/AP-1 activation through TLR/MyD88 and protective effects in the intestinal barrier |
| Wang et al., 2018 [159] | Hippophae rhamnoides L. berries pectin | In vivo | Cyclophosphamide induced immunosuppressive mice | Macrophage activation, MyD88 increased expression and upregulated expression of TLR4 |
| Park et al., 2013 [160] | RG-II from P. ginseng | In vivo and In vitro | C57BL6 WT, TCR KO, TLR KO mice, and BMDC cells | Facilitation of CD8+ T cells, induced production of TNF-α, IL-12, IFN-γ, and IL-1β during dendritic cell maturation |
| Sahasrabudhe et al., 2018 [161] | Lemon pectins with different DM | In vitro and In vivo | HEK-Blue WT and mutated cell lines, female C57BL/6 mice | Inhibition of TLR2-1 heterodimer, prevention of ileitis in the mice model |
| Hu et al., 2021 [162] | Lemon pectins with different DM | In vivo | Sprague-Dawley male rats and C57BL/6 mice | Reduced peri-capsular fibrosis in vivo and decreased DAMP-induced TLR2 immune activation in vitro |
| Kolatsi-Jannou et al., 2011 [163] | MCP | In vivo | Male C57BL/6J mice | Reduced Gal-3 expression, reduced renal cell proliferation, apoptosis, fibrosis, and proinflammatory cytokine expression |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrosa, L.d.F.; Raz, A.; Fabi, J.P. The Complex Biological Effects of Pectin: Galectin-3 Targeting as Potential Human Health Improvement? Biomolecules 2022, 12, 289. https://doi.org/10.3390/biom12020289
Pedrosa LdF, Raz A, Fabi JP. The Complex Biological Effects of Pectin: Galectin-3 Targeting as Potential Human Health Improvement? Biomolecules. 2022; 12(2):289. https://doi.org/10.3390/biom12020289
Chicago/Turabian StylePedrosa, Lucas de Freitas, Avraham Raz, and João Paulo Fabi. 2022. "The Complex Biological Effects of Pectin: Galectin-3 Targeting as Potential Human Health Improvement?" Biomolecules 12, no. 2: 289. https://doi.org/10.3390/biom12020289
APA StylePedrosa, L. d. F., Raz, A., & Fabi, J. P. (2022). The Complex Biological Effects of Pectin: Galectin-3 Targeting as Potential Human Health Improvement? Biomolecules, 12(2), 289. https://doi.org/10.3390/biom12020289